Chaperone gene therapy delays ALS
Despite its rather specific name, macrophage migration inhibitory factor (MIF) has multiple roles. One of those roles is to serve as a chaperone protein that helps the proper folding of superoxide dismutase (SOD1). In the 10% of amyotrophic lateral sclerosis cases that have a known genetic cause, SOD1 mutations are one of the largest subgroups, and previous work had shown that MIF could reduce the misfolding of mutated SOD1 in cell culture. In the current study, researchers from Ben-Gurion University of the Negev used gene therapy to deliver MIF to the brains of ALS mouse models, and showed that this treatment reduced SOD1 aggregation, delayed disease onset and extended survival in the animals. Their work appeared in the July 1, 2019, online issue of the Proceedings of the National Academy of Sciences.
Botox for – well, against – mosquitoes
Researchers from Stockholm University and the University of California at Riverside have identified a toxin produced by the bacterium Paraclostridium bifermentans that selectively killed Anopheles mosquitoes. Bacterially produced toxins have been used in biological parasite control and have been less resistance prone than chemical approaches, which are losing their effectiveness in antimalarial efforts. Clostridium bacteria produce the neurotoxins botulinum toxin and tetanus toxin, which target the vertebrate neuromuscular toxin, prompting the authors to search for related bacteria that could target insects. They showed that the soil bacterium Paraclostridium bifermentans subsp. malaysia produced a protein, Paraclostridial mosquitocidal protein 1 (PMP1), that killed Anopheles larvae by cleaving mosquito syntaxin. The authors concluded that "our research has an important impact on the study of the evolution of clostridial neurotoxins (CNTs) and provides the basis for the use of P. bifermentans strains and PMP1 as innovative, environmentally friendly approaches to reduce malaria through anopheline control. Broadening the range of CNTs specificity found in Clostridium and non-Clostridium species will be useful, not only for medical applications but also for novel biotechnological purposes." They reported their results in the July 2, 2019, issue of Nature Communications.
PTEN and CF
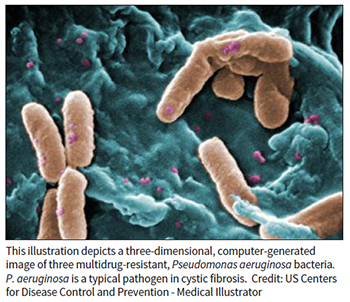 A Columbia University team has uncovered metabolic activity of PTEN as the molecular mechanism behind the increased susceptibility to Pseudomonas aeruginosa infections of cystic fibrosis (CF) patients. PTEN is best known as a tumor suppressor that plays a role in regulating cell proliferation, but it is widely expressed and plays multiple roles in physiology. One of those roles is the control of succinate metabolism in mitochondria. In their research, the authors showed that PTEN is associated with the cystic fibrosis transmembrane conductance regulator (CFTR). In cells with CF-causing mutations, that complex was functionally impaired and caused mitochondrial metabolism to change in a way that increased levels of succinate, which P. aeruginosa uses as fuel. The authors hypothesized that "the cycle of P. aeruginosa infection, adaptation, and establishment of intractable biofilms in the airway could be mitigated by early treatment to enhance the PTEN-CFTR interaction and prevent mitochondrial dysfunction" and its attendant increased succinate levels. They published their findings in the July 3, 2019, issue of Science Translational Medicine.
A Columbia University team has uncovered metabolic activity of PTEN as the molecular mechanism behind the increased susceptibility to Pseudomonas aeruginosa infections of cystic fibrosis (CF) patients. PTEN is best known as a tumor suppressor that plays a role in regulating cell proliferation, but it is widely expressed and plays multiple roles in physiology. One of those roles is the control of succinate metabolism in mitochondria. In their research, the authors showed that PTEN is associated with the cystic fibrosis transmembrane conductance regulator (CFTR). In cells with CF-causing mutations, that complex was functionally impaired and caused mitochondrial metabolism to change in a way that increased levels of succinate, which P. aeruginosa uses as fuel. The authors hypothesized that "the cycle of P. aeruginosa infection, adaptation, and establishment of intractable biofilms in the airway could be mitigated by early treatment to enhance the PTEN-CFTR interaction and prevent mitochondrial dysfunction" and its attendant increased succinate levels. They published their findings in the July 3, 2019, issue of Science Translational Medicine.
Disordered protein domains stay disordered at work
Researchers from Lehigh University and Brown University have found that low-complexity domains (LCDs) of proteins did not take on more definite structures when they formed aggregates. LCDs are regions of proteins that do not form into stable 3-dimensional shapes, but either cycle between multiple different shapes or remain a loose string of amino acids. Many RNA-binding proteins contain LCDs, and interactions between those LCDs cause the proteins to aggregate, which is important for their roles in RNA transport. Protein aggregates are also a key feature of neurodegenerative disorders, and understanding how LCD proteins aggregate could be important for understanding both their normal and their pathological functions. Previous work had suggested that LCDs can assemble into a structure called beta-pleated sheets, and that this might underlie their interactions in the aggregated state. However, the authors looked at aggregated LCDs using several methods and could find no evidence for interactions via beta-pleated sheet formation, finding instead that hydrogen bonding, hydrophobic interactions and pi/sp2 orbital bonding accounted for aggregation of the RNA-binding protein FUS, which is implicated in amyotrophic lateral sclerosis and frontotemporal dementia. The team published its findings in the July 1, 2019, issue of Nature Structural and Molecular Biology.
'Minor' subclones, via microenvironment, help cancer spread
Scientists at the Dana-Farber Cancer Institute have demonstrated that in heterogenous tumors, minor subclones could help other subclones spread via their effects on the tumor microenvironment. Heterogenous tumors that are made up of many genetically distinct subclones have a poorer prognosis than more homogenous ones, but what makes them worse news is largely unclear. In their work, the team demonstrated that in heterogenous breast cancers, subclones expressing interleukin-11 (IL-11) and Fos-induced growth factor (FIGF) affected the bone marrow stroma to promote metastatic seeding. The team also pinpointed neutrophils as a cell population that responded to IL-11, and showed that depleting neutrophils prevented metastatic outgrowth of heterogenous breast tumors in animal studies. Metastasis-promoting subclones accounted for only 1% to 5% of total tumor cells, and so "their presence and their secreted factors may be difficult to detect in clinical samples, making it challenging to predict their metastasis-promoting effects in human tumors," the authors acknowledged. "Nevertheless, our analysis of human metastatic breast tumors suggests that the systemic changes in immune and other bone-marrow-derived cells, which are induced by even low levels of these secreted factors, can be detected and potentially exploited for patient stratification and treatment." They reported their results in the July 1, 2019, issue of Nature Cell Biology.
New insights into old Fountain of Youth
Stem cells may be considered the fountain of youth, but even stem cells grow old. Researchers at Stanford University have used single-cell analysis of cells from the subventricular zone, one of the few brain areas where new neurons continue to be born in adult animals, to understand what happens when they do. The authors showed that T cells infiltrated the subventricular zone with aging, and secreted interferon-gamma that impaired the function of neural stem cells (NSCs). The authors also noted that the infiltrating T cells were clonally expanded, "which suggests that they may recognize a specific antigen in the old brain," the authors wrote. They concluded that "although the exact link between T cells, interferon and NSC proliferation remains to be established, our results provide a possible cause for the decline of NSC during ageing and suggest avenues through which to counteract age-associated cognitive impairment." Their work appeared in the July 4, 2019, issue of Nature.
Naïve B cells have tell-tale signature in lupus
Researchers at Emory University have used comparative profiling of different types of B cells to show that in systemic lupus erythematosus (SLE, lupus), "an SLE molecular signature was already established in resting naive cells." The production of autoantibodies is a dominant feature of lupus, and genetic risk factors for the disease are concentrated in the B-cell receptor. Previous studies had also shown that the epigenetics of B cells are altered in lupus, prompting the authors to compare "DNA methylation epigenomes, chromatin accessibility profiles and transcriptomes of human B cells from healthy controls and subjects with SLE, analyzing cell subsets that represent resting, activated and memory compartments." The team showed that though B cells from healthy individuals and SLE patients were similar in terms of their gene expression profiles, the B cells from SLE subjects were "epigenetically primed" to be activated more readily than those from controls. The team reported its results in the July 1, 2019, issue of Nature Immunology.
Pre-clearance boosts immunotherapy in pancreatic cancer
Scientists at Washington University in St. Louis have shown that checkpoint blockade's effects could be boosted in animal models of pancreatic cancer through treatment with a compound targeting myeloid cells. Myeloid cells, like many cell types, can have pro- or antitumor effects, and in their studies, the authors showed that an agonist of the myeloid surface protein CD11b shifted those cells to an antitumor state in the microenvironment of pancreatic tumors. That shift, in turn, provided a boost to T-cell activation via PD-1 blockade. "Our data highlight a promising strategy to target multiple lineages of immunosuppressive myeloid cells with a single agent that activates CD11b signaling," the authors wrote. "Our data demonstrate that the successful reprogramming of the innate immune compartment by CD11b agonism can render [pancreatic] tumors more sensitive to checkpoint blockade." Their work appeared in the July 3, 2019, issue of Science Translational Medicine.
New target improves metabolism
In metabolic dysfunction, multiple physiological and cellular variables correlate with each other, and with poor health outcomes. Teasing out the cause and effect relationship between those multiple variables, let alone how to successfully manipulate them to improve health, has been an ongoing challenge, but ceramides and dihydroceramides, two related types of lipids, have been shown to correlate closely with insulin resistance. Now, a team from the University of Utah has shown that preventing the conversion of dihydroceramides into ceramides by targeting the enzyme dihydroceramide desaturase-1 (DES1) improved lipid and glucose metabolism in mice. "Ablation of DES1 from whole animals, or tissue-specific deletion in the liver, and/or adipose tissue resolved hepatic steatosis and insulin resistance in mice caused by leptin deficiency or obesogenic diets. Mechanistic studies revealed new ceramide actions that promoted lipid uptake and storage and impaired glucose utilization, none of which could be recapitulated by (dihydro)ceramides that lacked the critical double bond," the authors wrote. "These studies suggest that inhibition of DES1 may provide a means of treating hepatic steatosis and metabolic disorders." Their work appeared in the July 4, 2019, online issue of Science.

