Histone deacetylases (HDACs), the cellular enzymes whose functions include turning gene expression off and on, are promising targets in current drug development for cancer therapy. While treatment with HDAC inhibitors as monotherapies has demonstrated clinical benefit for patients with various hematological and solid tumor malignancies, there is excitement surrounding early results of their use together with other cancer therapeutics. That has spurred an increase in the research and development of treatment combinations with therapeutics such as checkpoint inhibitors.
Lexington, Mass.-based Curis Inc., for example, is developing its CUDC-907 – a synthetic, orally available, small molecule that has the potential to inhibit the activity of HDAC – combined with phosphatidylinositol 3 kinase (PI3K) inhibitors. In June, the company updated data from a phase I trial of CUDC-907 in 75 patients with relapsed/refractory lymphoma or multiple myeloma at the European Hematology Association's annual meeting. Data from 21 response-evaluable patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) showed objective responses reported in nine of them, including three patients with complete responses. Additionally, a retrospective post-hoc analysis showed that among six response-evaluable DLBCL patients whose tumors were characterized with MYC alterations, five experienced objective responses, including three patients with complete responses. All five patients with MYC-altered disease who experienced objective responses also had alterations in BCL-2, including two patients with BCL-2 gene translocations.
The company is currently enrolling relapsed/refractory DLBCL patients in a phase II monotherapy trial of CUDC-907 to assess its efficacy specifically in patients with MYC-altered DLBCL. Data from are expected next year.
Analyst Chris Shibutani at Cowen and Co. said he believes CUDC-907 has the potential to advance relatively quickly.
Expanding pipeline
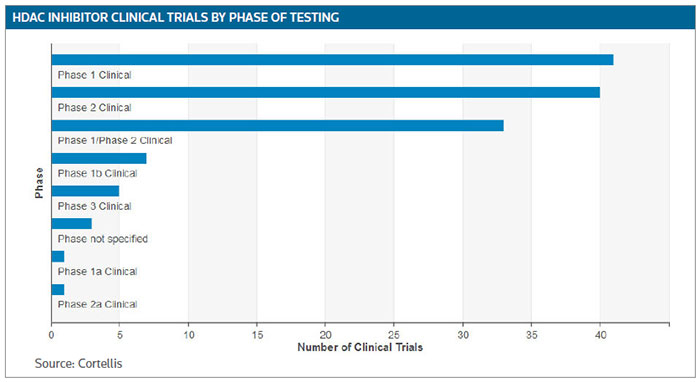
Clinical development is growing and there are approximately 130 trials that are currently recruiting or in the planning stages, according to Cortellis Clinical Trials Intelligence. (See HDAC inhibitor clinical trials by phase of testing, below.)
Syndax Pharmaceuticals Inc., which completed its IPO in March, is collaborating with Merck KGaA, of Darmstadt, Germany, and Pfizer Inc. to evaluate avelumab, an investigational fully human anti-PD-L1 IgG1 monoclonal antibody, in combination with Syndax's entinostat, an oral HDAC inhibitor, in patients with heavily pre-treated, recurrent ovarian cancer. Syndax will be responsible for conducting a phase Ib/II trial.
Speaking on the importance of the study, Luciano Rossetti, head of global research and development of the biopharma business of Merck KGaA, said, "Combination therapy is the next frontier in immuno-oncology and a key strategy for the alliance."
Syndax also has a clinical collaboration with Genentech Inc., a unit of the Roche Group, of Basel, Switzerland, to evaluate entinostat in combination with atezolizumab (MPDL3280A), a fully humanized monoclonal antibody (MAb) targeting PD-L1 in patients with triple-negative breast cancer. (See BioWorld Today, March 4, 2016.)
In a phase III program, the company is testing a entinostat in advanced HR-positive breast cancer, and proceeds from the recent IPO will help support those ongoing trials, the filing of a new drug application and manufacturing of registration batches of active pharmaceutical ingredient and final drug product. The company plans to use other funds from the offering to support various combination trials.
The company said it has completed enrollment for a dose confirmation stage of ENCORE 601, a phase Ib/II trial evaluating the combination of entinostat plus Merck & Co. Inc.'s anti-PD-1 blocking therapy, Keytruda (pembrolizumab), in patients with advanced metastatic or recurrent non-small-cell lung cancer (NSCLC) or melanoma. It is moving ahead with the phase II portion of the study.
San Diego-based MEI Pharma Inc. attracted Swiss pharma Helsinn Group for an exclusive licensing, development and commercialization agreement for HDAC inhibitor pracinostat, a phase III-ready candidate for the treatment of acute myeloid leukemia (AML) and other potential indications. (See BioWorld Today, Aug. 9, 2016.)
A phase III study is expected to start early next year and will take about two and a half years to enroll approximately 525 patients, mostly in the U.S. and Western Europe, with a primary endpoint of overall survival based on an analysis about a year and a half after the last patient is enrolled.
At the beginning of this month, the company reported that the FDA had granted breakthrough therapy designation for pracinostat in combination with azacitidine for the treatment of patients with newly diagnosed AML who are 75 or older or who are unfit for intensive chemotherapy. The designation was supported by data from a phase II study, which showed a median overall survival of 19.1 months and a complete response (CR) rate of 42 percent (21 of 50 patients). Those data compare favorably to a phase III study of azacitidine (AZA-AML-0011), which showed a median overall survival of 10.4 months with azacitidine alone and a CR rate of 19.5 percent in a similar patient population.
Collaborations
Mirati Therapeutics Inc., of San Diego, initiated a trial for the combination study of mocetinostat, an HDAC inhibitor, with the Astrazeneca/Medimmune anti-PD-L1 checkpoint inhibitor, durvalumab, in patients with NSCLC. That trial is exploring the potential of mocetinostat to enhance the effectiveness of checkpoint inhibitors in NSCLC. The company said it believes the dual effect of class I HDACs on tumor cells, as well as on immune cells, may enhance the effect of checkpoint inhibitors in all indications where checkpoint inhibitors have demonstrated efficacy.
Mirati struck a deal with Astrazeneca/MedImmune last year in which it will conduct and pay for the first phase I/II study in NSCLC, with London-based Astrazeneca's Medimmune arm providing supply of durvalumab. Medimmune holds an option to negotiate a license if the experiment succeeds. (See BioWorld Today, Aug. 6, 2015.)
In June, Boston-based Acetylon Pharmaceuticals Inc. presented positive results from multiple clinical trials evaluating the safety and efficacy of two selective HDAC6 inhibitors in combination with pomalidomide (Pom) (Pomalyst, Celgene Corp.) and dexamethasone (Dex) for the treatment of relapsed or relapsed-and-refractory multiple myeloma at the European Hematology Association meeting. The results of the phase II ACE-MM-102 trial indicated that treatment with ricolinostat in combination with Pom and Dex was very well-tolerated, and toxicities were predominantly low grade. Pharmacokinetic and pharmacodynamic analysis demonstrated selective inhibition of HDAC6 at therapeutic doses. Analysis of 67 efficacy-evaluable patients enrolled at least six months prior to the data cut confirmed an overall response rate of 46 percent, a clinical benefit rate of 58 percent, nine months duration of response and seven months progression-free survival.
In 2013, the company signed a strategic collaboration agreement with Celgene Corp., which includes an exclusive option for the future acquisition of Acetylon.
The company also has on ongoing partnership with the Hereditary Neuropathy Foundation (HNF), of New York. As a supplement to HNF's longstanding collaboration with University of Sheffield, Acetylon will provide an HDAC6 inhibitor compound for testing in a preclinical model of Charcot-Marie-Tooth, the most common inherited peripheral neuropathy.
Huya Bioscience International LLC is working with Japanese pharmaceutical company Eisai Co. Ltd. for the commercialization of its HBI-8000 class I-selective oral HDAC inhibitor, approved in China for the treatment of peripheral T-cell lymphoma. (See BioWorld Today, Feb. 3, 2016.)
HBI-8000 inhibits class I HDAC1, HDAC2, HDAC3, as well as class IIb HDAC10 and stimulates accumulation of acetylated histones H3 and H4 in tumor cells.