To read more on these records and others in the pharmaceutical arena, please visit Clarivate Analytics Cortellis, where you may access full pdf documents of the same, along with expert analysis and indexing of their content to accompany detailing of such aspects as expirations, infringements, licensing and exclusivity.
The Patent Gazette provides snapshot analysis and indexing of pharmaceutically relevant patenting within days of its publication by patent offices. Primarily focusing on material from the main three patents offices (i.e., the EPO, USPTO, and WIPO), it provides brief descriptions of a patent’s content and seeks to link it to both prior patenting of relevance and to any commercial activity pertinent to the technology being described.
Subscription and access to Cortellis may be tailored and restricted to particular subject areas of interest, such as only its patenting content. For more details please do contact us.
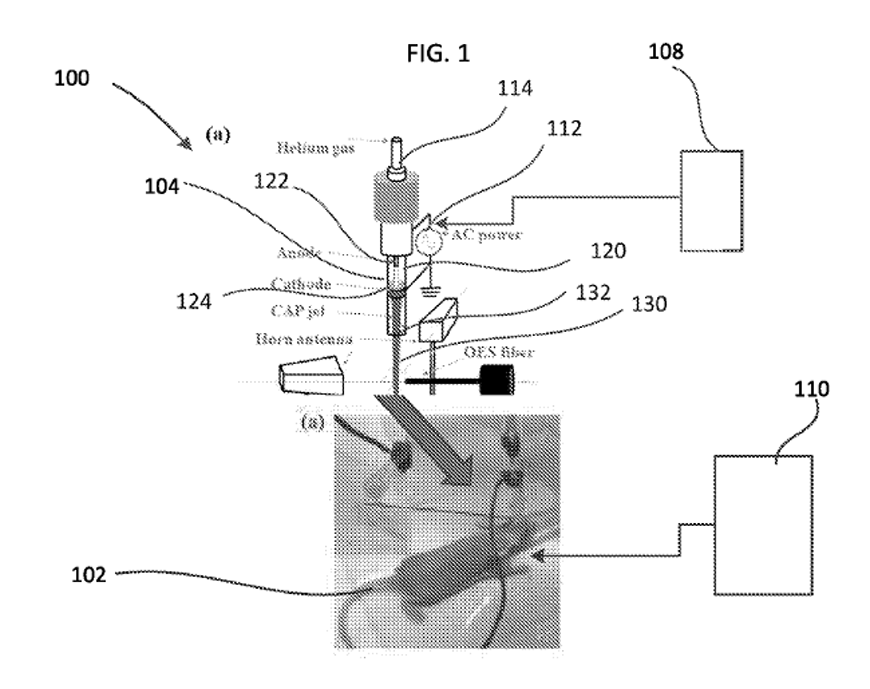
WO2019232438-A1: “Method and system of sensitizing cancer cells to chemical treatment by plasma based activation.”
Assignee: Gjika, Eda; Keidar, Michael; Yan, Dayun
Inventors: Gjika, Eda; Keidar, Michael; Yan, Dayun
IPC Codes: A61B 18/18; A61B 18/04; A61B 18/02
Publication Date: 05-Dec-2019
Earliest Priority Details: US2018679174, 01-Jun-2018
A novel process to sensitize cancer cells to a series of chemicals from a cold atmospheric plasma (CAP) treatment in a carrier gas such as helium. CAP is a near-room temperature ionized gas composed of electrons, neutral particles, charged particles, and reactive species. Included are the results of an in vitro study demonstrating the efficacy of the non-thermal plasma technology in combination with temozolomide treatment in a chemotherapy resistant glioblastoma cell line (U87MG).
This application comes not long after the granting of US10479979-B2 in November 2019, in which the inventors Keidar and Yan described a method for making and using CAP stimulated media for cancer treatment.
Professor Michael Keidar of the George Washington University was one of the first researchers to develop a CAP generator, and by 2011 he had published a paper in the Journal of Cancer showing that CAP selectively kills cancer cells in animal models. He began consulting with US Medical Innovations (USMI) in 2013 on creating an industrial-scale prototype of the CAP generator and its application for cancer treatment, based on his patented generator. The goal was to integrate CAP with Canady Hybrid Plasma™ electrosurgical scalpels, which simultaneously cut and coagulate tissue, sealing off blood vessels. USMI is a Biomedical and Life Science subsidiary of US Patent Innovations LLC for whom Keidar described the integration of a high frequency electrosurgical generator and plasma gas to deliver CAP (Plasma Helium Beam) for the selective treatment of cancer in US9999462-B2 (issued June 2018).
At the very end of July 2019, it was announced that the US FDA had approved the first clinical trial in the US to evaluate their CAP technology for treating cancer.
WO2019232291-A1: “Drug delivery articles for gram-level dosing.”
Assignee: Brigham & Women’s Hospital; Massachusetts Institute of Technology
Inventors: Cima, Michael, J.; Langer, Robert, S.; Roxhed, Niclas; Steiger, Christoph, Winfried Johannes; Traverso, Carlo, Giovanni; Verma, Malvika; Vishwanath, Karan
IPC Codes: A61K 31/4409; A61M 25/01; A61B 17/50; A61M 31/00; A61K 9/28; A61K 31/65; A61M 25/00; A61K 9/00; A61K 9/20; A61B 17/52; A61K 31/4709; A61K 31/496; A61K 31/133; A61K 31/4965
Publication Date: 05-Dec-2019 (shares priority details with co-published WO2019232292-A1, ‘296-A1, US20190366064-A1, ‘5645-A1, and ‘5418-A1)
Earliest Priority Details: US2018678439, 31-May-2018
Gastric resident system (GRS) capable of transesophageal administration, transesophageal retrieval, and/or gastric retention. The GRS is said to be capable of eg one-time administration through a nasogastric or endoscopic tube, safe retention in the gastric cavity, providing two weeks of drug release, and/or one-time transesophageal retrieval through a nasogastric or endoscopic tube. In one embodiment the GRS consists of a series of drug pills on a coiled superelastic nitinol wire and the retrieval device consists of a Hall effect sensor and a magnet that can detect and attach to the magnets on either end of the GRS.
Published alongside WO2019232292 and WO2019232296, describing similar GRS articles. Langer (the David H. Koch Institute Professor at MIT and a member of the Koch Institute for Integrative Cancer Research) and Traverso (an Assistant Professor in MIT’s Department of Mechanical Engineering and a gastroenterologist at Brigham and Women’s Hospital) have previously developed several novel strategies for oral delivery of drugs that usually have to be injected. Those efforts include a pill coated with many tiny needles, as well as star-shaped structures that unfold and can remain in the stomach from days to weeks while releasing drugs. In WO2019222570 they can be seen to have described self-righting capsules with tissue engaging surfaces.
The two inventors also gained media attention in October 2019, with it being reported how while working with scientists from Novo Nordisk, they had designed a new drug capsule that can carry insulin or other protein drugs and protect them from the harsh environment of the gastrointestinal tract. When the capsule reaches the small intestine, it breaks down to reveal dissolvable microneedles that attach to the intestinal wall and release their drug payload for uptake into the bloodstream.
WO2019232242-A1: “Methods for promoting hair growth.”
Assignee: Follica Inc
Inventors: Bhardwaj, Jason, Venkat; Bissett, Jonathan, Lee; Chastain, David, Paul; Washenik, Ken
IPC Codes: A61P 17/14; A61M 37/00; A61Q 7/02
Publication Date: 05-Dec-2019
Earliest Priority Details: US2018678068, 30-May-2018
Methods for stimulating hair growth in area of hair loss associated with male or female pattern baldness, androgenetic alopecia (AGA), primary cicatricial alopecia, or Lichen Planopilaris (LPP), by use of a reciprocating microneedle device to disrupt the area of skin to be treated followed by the application of a solution of minoxidil. Included within the invention’s examples are the results of clinical studies to assess the safety and efficacy of treatment methods using a needling device in conjunction with minoxidil in adults with AGA, female pattern baldness, and LPP/scarring alopecia.
Follows claims from one of the team, Chastain, describing a needling device and drug applicator in WO2017054009 (a family member of which, US20180280675, is referenced in the present application’s disclosure). Also see WO2019055662, in which Bhardwaj described similar methods for promoting hair growth but using valproic acid in place of minoxidil.
Boston, Massachusetts-based Follica’s proprietary investigational device is designed to induce an embryonic window via a device that creates micro-abrasions and initiates hair follicle neogenesis. In June 2019, Follica announced positive interim data from an ongoing safety and optimization study to treat hair loss in male AGA. In addition to being well tolerated and informing key treatment parameters, analysis of 20 male study participants with AGA showed that Follica’s approach achieved a visible and statistically significant improvement in non-vellus (visible) hair count after three months of treatment, compared to baseline. The company added that a pivotal study was expected to initiate at the end of 2019, subject to continued safety and efficacy in its optimization study.
WO2019229563-A1: “Automated smart watch assistance in the event of cardiac arrest.”
Assignee: IBM United Kingdom Ltd; International Business Machines Corp
Inventors: Cunico, Hernan; Frank, Paul, Alexander, Raphael; Keen, Martin; Smye-Rumsby, Adam
IPC Codes: A61B 5/021; A61B 5/01; A61B 5/02; G08B 21/04; A61B 5/00
Publication Date: 05-Dec-2019 (also published as US20190365333-A1)
Earliest Priority Details: US2018992327, 30-May-2018
It is discussed how smart watches can assist in situations of cardiac arrest where cardiopulmonary resuscitation (CPR) is needed to revive a person. Smart watches it is said may detect the impending presence of a heart related illness through the monitoring of a user’s heart rate using sensors embedded within the smart watch and may issue an alert if a heart related illness is detected. The smart watch may also assist in the act of performing compression CPR by using sensors on the smart watch to monitor the movements a user makes during CPR administration and to ensure that the user is performing compression CPR at the correct pressure and rhythm.
The broadcasted CPR alert (eg text message) may include profile information of the user in distress, location information, and other relevant information of the user in distress. The broadcasted CPR alert may also be sent over ultrasound communication using an onboard speaker of a user’s smart watch. Although users of the automated smart watch assistance program may not sense the ultrasound communication, the transmission may contain data encoded in ultrasound concerning the nature of the alert, including the location of the victim, the victim’s photograph and the victim’s name. The inventors say such elements as the location of the victim, the victim's photograph, and the victim's name may be used by an assisting user to locate a victim in a densely populated area. A victim’s photograph, used in combination with the location of the victim’s distress signal, the inventors say may enable immediate physical identification of a victim eg at a crowded concert by an assisting user.
While this represents a new patenting interest for the inventors, the approval of another of IBM’s smartwatch patent applications as US10319331-B2 in June 2019 was seen to gain some media attention at that time, with some calling the invention the weirdest smartwatch ever - with its diagrams being seen to illustrate a watch device with a screen that can then flip out to become a smartphone-sized display, which can then in turn unfurl further to provide a tablet-like face.
WO2019232488-A1: “Venous infusion catheter and methods for its use.”
Assignee: Voyage Biomedical Inc
Inventors: Jansen, Tatiana M.; Jones, Melissa E. R.; Olshavsky, Justin M.; Schultz, Robert D.; Shaw, Aurko J.; Vaughan, Bridget C.
IPC Codes: A61M 25/10; A61M 39/02; A61B 18/24
Publication Date: 05-Dec-2019
Earliest Priority Details: US2018679242, 01-Jun-2018
Venous infusion catheters and a method for treating a patient by introducing a catheter into the patient’s venous vasculature to position a first outlet port on the catheter at a location where blood flows into the patient’s right atrium and a second outlet port on the catheter at a location where venous blood drains from the cerebral vasculature. One or more medicaments may be delivered in an antegrade direction to the right atrium of the patient’s heart through the first outlet port of the catheter at selected times. At other selected times, a preservative medium, such as a hypothermic fluid, may be delivered in a retrograde direction to the patient’s cerebral vasculature through the second outlet port of the catheter. In this way, the catheter may be used in a manner analogous to a central venous catheter for delivering drugs and other medicaments to the patient’s heart, eg in a hospital setting such as an intensive care unit. Should an in-hospital cardiac arrest (IHCA) or other emergency occur, such as a stroke, the catheter may then be used for the delivery of a cooling or other preservative medium to the patient’s cerebral vasculature.
Dr Robert Schultz is a cardiac surgery resident with a special interest in extracorporeal life support for cardiorespiratory failure at the Libin Cardiovascular Institute of Alberta in Canada. He is also founder and CEO of San Francisco, California-based Voyage Biomedical Inc and the creator of its Boreas Central Line (BCL) that is seemingly described within this patent application. The BCL is said to be a specialized central venous catheter that allows for administration of fluids to achieve targeted, deep, and rapid cooling of the brain and help prevent damage thereto during cardiac arrest.
WO2019229407-A1: “Evaluation of an amount of a substance contained within circulating blood.”
Assignee: Zedsen Ltd
Inventors: Mamigonians, Hrand Mami
IPC Codes: A61B 5/00; A61B 5/145; A61B 5/1468; A61B 5/053
Publication Date: 05-Dec-2019 (also published US20190365300-A1)
Earliest Priority Details: GB20188857, 31-May-2018
Apparatus and method for the non-invasive evaluation of an amount of a substance (eg glucose) contained within blood circulating within human body tissue. When a finger or other body part is placed onto a sensor present on the device, electrical fields pass through the tissue to produce monitored output data that through techniques of machine learning may be interpreted to provide an indication of blood glucose levels.
This patent application comes almost one month after the publication of WO2019211572 in which the same inventor described the use of the company’s technology for examining objects with electrical fields (see WO2019110949) for detecting irregularities in breast tissue.
Dr Hrand Mamigonians is co-founder and CTO of London, UK-based Zedsen that describes itself as a developer of an intelligent and flexible sensor technology aimed at a wide range of applications. The company’s technology is able to see into and through materials whether solid, liquid, powder or gas, sensing pressures and material composition at a distance, underpinned by advanced algorithms and machine learning capabilities that create novel imaging capabilities, enabling healthcare, life sciences, automotive, aerospace and security industries to get access to a transformative and innovative range of capabilities.
WO2019227203-A1: “Method and device for treating sleep related breathing disorders.”
Assignee: Zennea Technologies Inc
Inventors: Chase, Rachel; Du, Jia; Luo, Oliver; Threlfall, Ryan
IPC Codes: A61N 1/36; A61F 5/56
Publication Date: 05-Dec-2019
Earliest Priority Details: US2018679496, 01-Jun-2018
Methods and systems for treating sleep related breathing disorders by increasing airway patency and/or maintaining airway patency. Treatment takes the form of a small device, that is positioned on an individual’s neck, beneath their chin, that has four electrode stimulators for stimulating at least four regions of their airway. Using transcutaneous electrical nerve stimulation-based technology to target the hypoglossal nerve to contract the genioglossus muscle and the hyoglossus muscle, the styloglossus muscle, the superior longitudinal muscle, the inferior longitudinal muscle, the transverse muscle, the vertical muscle, or a combination thereof, this results in the opening of the upper airway and improvements in breathing.
Represents the first patenting from the Canadian assignee, Zennea Technologies Inc, a Simon Fraser University student venture that is based in Surrey, British Columbia. Co-founded by the inventors Chase, Du and Luo the company is seeking to develop what would be the first clinically proven medical device for snoring and mild obstructive sleep apnea to be approved by the US FDA for use when a CPAP (continuous positive airway pressure) machine would not be prescribed. As seen in this patent application, Zennea’s ZENS device uses neuro-stimulation technology that externally activates cranial nerves to contract the main dilator muscles of the tongue and reduce upper airway restrictions.
US20190365606-A1: “Method and system for autonomously measuring, recording, and reporting changes in the interior content of containers.”
Assignee: The Trustees of Indiana University
Inventors: Nezamuddin Omar N; Dos Santos Jr. Euzeli C; Tunnell iv Harry D; Bartlett Ellis Rebecca J; Hill James Haswell; Momoh Ibrahim Aigbodesi
IPC Codes: A61J 7/04; H04W 4/029; B65D 83/04; A61J 7/00; A61J 1/03
Publication Date: 05-Dec-2019
Earliest Priority Details: US2018680340, 04-Jun-2018
The application describes a system for tracking medication comprising a pillbox capable of monitoring the usage and content of the pillbox and relaying it to a remote device, particularly a phone app.
The application appears to describe the University's InterACT pillbox which forms part of Bartlett Ellis' Medication-taking Across the Care Continuum and Adherence-related Outcomes (MACO) Framework. Funding of $107,500 for pilot testing has been received.
The application appears to be the first from the team.
WO2019232143-A1: “System and method for collecting and displaying data acquired from an implantable therapy device using a consumer electronic device.”
Assignee: Inspire Medical Systems Inc
Inventors: Rondoni, John; Dieken, David, Todd
IPC Codes: A61N 1/372
Publication Date: 05-Dec-2019
Earliest Priority Details: US2018678665, 31-May-2018
The application describes a method for establishing communication between a patient-implantable device and an electronic device operable by the patient. The method allows the patient to control the implanted device.
The application appears to be targeted towards a controller for the company's FDA-approved sleep apnea treatment which opens the airway by restoring muscle tone. The STAR clinical trial showed that this device reduced AHI by 79% and produced significant improvements in daytime functioning and reductions in daytime sleepiness.
See also the copublished WO2019231822 on a method for aggregating patient data and WO2019231821 on methods for the secured sharing of patient data. The present application follows from WO2016168119 on a method for monitoring patient-made changes in stimulation therapy.
WO2019232450-A1: “Detection of biological substances.”
Assignee: Orb Xyz Inc
Inventors: Falzarano, Lorenzo; Smith-Moritz, Andreia, Michelle
IPC Codes: G01N 15/14; G01N 21/64; G01N 21/53
Publication Date: 05-Dec-2019
Earliest Priority Details: US2018679603, 01-Jun-2018
The application describes a method for detecting a biological substance in a fluid by exposing the substance to deep UV light and detecting then analyzing the emission produced by the excited biological substance. The method is envisaged for use in a wide variety of situations including detecting bacteria in water, or proteins or pregnancy markers in urine.
See also WO2019232448 which describes the corresponding system. These appear to be the first applications from the company.
WO2019232265-A1: “Drug delivery methods targeting the lymphatic system.”
Assignee: Sorrento Therapeutics Inc
Inventors: Ross, Russell Frederick
IPC Codes: A61K 9/00; A61M 37/00
Publication Date: 05-Dec-2019
Earliest Priority Details: US2018678584, 31-May-2018; US2018678592, 31-May-2018; US2018678601, 31-May-2018
The application describes a method for administering drug to multiple regions of the lymphatic system using microneedle arrays, specifically the SoFusa system. The application appears to be particularly directed towards the administration of etanercept and anti-mCTLA-4 monotherapy (BioXcell clone 9H10).
In July 2018, Sorrento acquired the Sofusa lymphatic delivery technology platform from Kimberley-Clark. It consists of nano-structured microneedles designed to access the lymphatic capillaries just below the epidermis.
This appears to be the first application from Sorrento on this technology.
US20190366035-A1: “System and method for bi-directional fluid injection.”
Assignee: Individual
Inventors: Sotolongo Alex
IPC Codes: A61M 25/00; A61M 25/01; A61M 1/36
Publication Date: 05-Dec-2019
Earliest Priority Details: US2018678286, 31-May-2018
The application describes a system and method for bi-directional fluid injection comprising a retrograde cannula in fluid connection with an antegrade cannula. In use fluid enters the retrograde cannula, a portion is diverted out of the antegrade cannula and the remaining fluid leaves via an outlet of the retrograde cannula.
The inventor appears to be new to patenting and to be associated with Angiio LLC and DualFlo LLC.

