CDU Contributing Editor
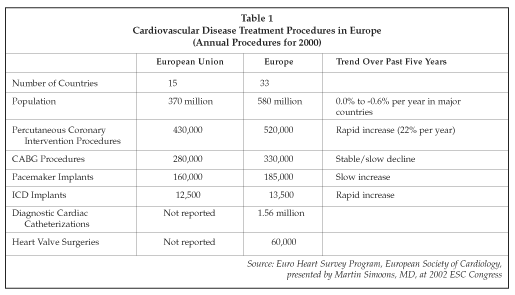
BERLIN, Germany – The 2002 Congress of the European Society of Cardiology (ESC; Sophia Antipolis, France), attended by more than 20,000 physicians, researchers and exhibitors, provided a venue for discussion of the latest developments in diagnostic and interventional cardiology for the European market. Heart disease is the leading cause of death in Europe as a whole, and while mortality rates for cardiovascular disease have decreased in most western European countries, due to expanded use of prevention strategies and better treatment, coronary heart disease mortality in the middle age groups is increasing rapidly in most of the countries in Eastern Europe. The number of procedures performed to treat cardiovascular disease in Europe is continuing to increase, although different types of procedures are exhibiting different trends. Percutaneous coronary interventional procedures, for example, totaled 430,000 in the European Union countries and 520,000 in Europe as a whole in 2000, as shown in Table 1, and growth is continuing at a rate of more than 20% per year. The use of implantable cardioverter defibrillators (ICDs) is also growing rapidly, although the total number of procedures remains small. Implants of pacemakers are also growing, but the rate of growth is slower than for percutaneous interventions and ICD implants, according to data presented at the ESC congress by investigators from the Euro Heart Survey.
 |
In contrast to trends for percutaneous intervention, the number of coronary artery bypass graft procedures performed annually in Europe has stabilized at about 330,000, and is expected to decline in most countries as more patients with coronary heart disease are treated with percutaneous intervention (PCI). The trend is expected to accelerate somewhat due to the introduction of drug-eluting coronary stents, providing outcomes equivalent to surgery in many patients and resulting in more patients choosing percutaneous intervention (PCI) over open surgery. A high percentage of patients (estimated at up to 90% in some countries) now receive stents, although at least at present restenosis rates of 20% to 30% for stents without drug-eluting capability contribute to a significant and growing population requiring re-intervention. Nevertheless, stents have proven effective in reducing myocardial events, cutting the incidence of heart attacks by 45%. The utilization rate of percutaneous intervention in Europe still lags behind that in the U.S. by a considerable factor (about 2,800 procedures per million inhabitants in the U.S. vs. only about 900 per million in Europe in 2000). As shown in Table 2, there also is considerable variation across Europe in the rate of PCI use between countries, from 2,200 per million in Germany to 100 per million in Romania. However, the PCI rate is increasing rapidly in all countries in Europe, and in the long term is expected to continue to approach the U.S. rate, although it probably will not reach that level. The trends in utilization, coupled with increasing prices for coronary stents as drug-eluting stents enter widespread use, will drive strong growth in the market for interventional cardiology products in Europe.
 |
Drug-eluting stents are now used in about 5% to 7% of patients undergoing stent implantation in high-utilization countries such as Germany. Only one drug-eluting stent, the Cypher stent from Cordis/Johnson & Johnson (Miami Lakes, Florida), is currently available, but many others are under development. Other new developments in interventional therapy include embolic protection devices, devices for the detection of vulnerable plaque, devices to enhance the efficacy of thrombolysis, such as the Intravascular Sonotherapy catheter from Pharmasonics (Sunnyvale, California), and devices to allow treatment of chronic total occlusions, including the Safe-Steer device from IntraLuminal Therapeutics (Carlsbad, California). Implantable defibrillators represent another category of products exhibiting rapid growth in the European market, along with devices for biventricular pacing in heart failure patients. Finally, certain emerging technologies promise to drive additional change in the market in the longer term, including tissue engineering technology to treat heart failure and coronary artery disease.
Drug-eluting stents continue to advance
The most recent data on the performance of drug-eluting stents continues to demonstrate that the devices can provide a long-term solution to the problem of restenosis, but in some patients the efficacy, while improved as compared to uncoated stents, leaves room for improvement. The types of patients treated in the RAVEL study, the initial trial of the Cypher stent that showed an almost total elimination of restenosis, represent only about 15% to 20% of the patients encountered in routine practice. The other 80% to 85% of patients with more complex lesions will likely prove more challenging to treat. For example, as discussed at ESC by Ron Waksman, MD, of Washington Hospital Center (Washington), an analysis of a subset of patients with complex lesions in the RAVEL study using the Cordis Cypher stent showed a restenosis rate of 10.5%. However, in diabetic patients, who represent another difficult patient group, the Cypher stent appears to be very effective, at least for patients with less complex lesions, with no significant change observed in post-treatment lumen diameter over a period of six months. Nevertheless, most users of drug-eluting stents believe that additional improvement is needed to deal with issues such as edge effects (i.e., restenosis in the region outside the ends of the stent); lack of apposition of the stent struts to the vessel wall in some cases; and high cost. In addition, the performance of drug-eluting stents has not yet been evaluated in chronic total occlusions, bifurcation lesions and left main coronary artery disease.
Cost is a particularly significant issue, in the opinion of many European cardiologists. The average cost of a coronary stent ranges from EUR 300 to EUR 500 in Germany up to about EUR 600 in the UK, according to presenters at the ESC congress. However, pricing for the Cypher drug-eluting stent ranges from about EUR 1,900 to EUR 2,300 in those countries. Even in relatively affluent countries such as Germany, many cardiologists believe those price levels make the devices unaffordable except for a small percentage of patients. However, there is no consensus as to how low the price must be in order to allow drug-eluting stents to be used for all patients who would benefit.
The cost issue is likely to be addressed over time as more suppliers enter the market with drug-eluting stents. As shown in Table 3, at least 15 new devices are at varying stages of development for the European market. Products expected to enter the market in Europe over the next year include the Taxus stent from Boston Scientific (Natick, Massachusetts), the Achieve from Guidant/Cook (Indianapolis, Indiana) and the Dexamet from Abbott Laboratories (Abbott Park, Illinois). A number of additional devices should be ready for launch by late 2003 or early 2004. As has occurred with existing coronary stents, prices are likely to remain high as long as only a single supplier is in the market, and they probably will not drop significantly even when a second or third device is introduced. However, in the long term, as multiple devices become available with competitive features, prices could drop to under $1,000.
 |
The characteristics of drug-eluting stents are designed to deal with the four key factors that are responsible for restenosis, including the inflammation that typically occurs upon initial implant due to a foreign body reaction in the vessel; elastic recoil (which can be prevented as long as the stent has sufficiently high radial strength); smooth muscle cell proliferation; and lack of endothelial cell coverage of the inner lumen of the stent, which leads to platelet adhesion and eventual thrombosis and occlusion of the lumen. In addition, balloon injury to regions outside the ends of the stent needs to be addressed, since the eluted drug does not penetrate to a significant extent past the edges of the stent. Improved implantation techniques should address that issue via the use of stents that completely cover the region exposed to stress in the initial balloon pre-dilatation. An important parameter, perhaps even more important than the drug used, is the degree of control and appropriateness of the level of drug released. Achieving the proper dosage level has proven to be the key challenge in the development of the Taxus stent, for example. As can be seen from the data in Table 3, a wide variety of approaches are being investigated for control of drug elution.
Another important area of development focus for suppliers of interventional cardiology products in Europe is embolic protection devices. Embolic debris can be released into the vessels by plaque rupture, or as a result of percutaneous intervention or surgery, potentially resulting in blockage of downstream blood flow. The results can include arrhythmias, cardiogenic shock, myocardial infarction, stroke, and heart failure. As discussed by G. Thiene, MD, of Padua, Italy, at the ESC congress, studies have demonstrated that microembolic debris is present in 83.7% of carotid interventional cases, with a mean particle diameter of 250 microns, although some debris particles were as long as 5 mm. Other studies indicate that distal embolization occurs in at least 15% of all percutaneous interventions. One device, the GuardWire Plus from Medtronic PercuSurge, has been on the market in Europe since 1999, and uses a balloon technology to block the migration of embolic material during a procedure, followed by aspiration of the material prior to re-establishing flow. A conclusive benefit was demonstrated for the device in the SAFER trial, and the product is now approved for sale in the U.S. However, studies have demonstrated that some embolization still occurs, probably as a result of device manipulation prior to deployment.
Another device now available in Europe is the TriActiv balloon-protected flush extraction system from Kensey Nash (Exton, Pennsylvania), priced at EUR 1,200. A console/pump system used with the device is supplied free of charge. A 42% drop in the rate of embolization has been observed for the TriActiv. The existing version is an 8 Fr device, but a new 7 Fr version is under development.
Physicians who have worked with various embolic protection devices generally find them somewhat difficult and time-consuming to deploy, and a residual level of embolization is still observed in most cases. A number of companies have developed alternative devices for embolic protection for sale in Europe. As discussed by Dietrich Baumgart, MD, of Essen, Germany, one of the more promising new devices is the CardioShield, developed by MedNova (Galway, Ireland) and distributed by Abbott Vascular. The CardioShield is a third-generation device with a .018" crossing profile that uses a polyurethane filter with 140 micron pores over a nitinol frame. A unique feature of the device is its use of a separate .014" wire that is first passed through the lesion to be treated, providing a stable and defined path for subsequent delivery of the device. The design also allows the filter to stay in place during wire manipulations in subsequent parts of a procedure, and maintains TIMI3 flow throughout the procedure. Two other versions, the EmboShield (for use in saphenous vein grafts) and NeuroShield (for carotid artery procedures), also are available. The average selling price for the Abbott/MedNova devices ranges from $900 to $1,000. Another new device, the Interceptor, is under development by Medtronic Vascular, and features a nitinol filter deployed in a stretched configuration, allowing initial deployment to be performed with minimal risk of dislodging debris during the process. Velocimed (Maple Grove, Minnesota) is developing the Proxis device, which uses two balloons to seal the vessel and stop blood flow in the region to be treated along with an evacuation sheath for debris removal.
Continued improvements in device technology are expected to stimulate additional growth in use of percutaneous interventional techniques in Europe over the next few years. In addition, recent studies discussed at the ESC meeting, particularly studies of the use of percutaneous intervention as a primary treatment modality for myocardial infarction patients, show that patients are likely to benefit from greater use of interventional therapy as opposed to more conservative medical treatment or thrombolytic therapy only. An important study, PRAGUE 2, described by Petr Widimsky, MD, of Prague, Czech Republic, evaluated the treatment of 429 patients with myocardial infarction who were first treated with thrombolysis in community hospitals because of the lack of catheterization facilities, and were then transported to other hospitals with a cath lab for percutaneous intervention. Overall, mortality was reduced to 6.8% for patients treated with percutaneous intervention vs. 10% for patients in a control group who were treated with thrombolysis only.
For patients who presented at the hospital more than three hours after onset of symptoms, the benefit of PCI was even greater, with mortality reduced to 7% vs. 15% in controls. The researchers said that the best approach is probably to take patients directly to a hospital with a cath lab facility. Transport proved to be relatively safe, with three deaths and three cases of ventricular fibrillation occurring in the 429 patients who were transported, even though the mean time to revascularization was in excess of four hours (277 minutes). According to Widimsky, only about 20% of myocardial infarction patients in Europe are treated with primary angioplasty at present, indicating a considerable potential for increased use of the approach.
New approaches to treating diseased saphenous vein grafts are also showing promise, allowing improved therapy of patients who have had prior bypass surgery and subsequently present with a stenosis of the graft. A new device from Boston Scientific, the Symbiot covered stent, has been evaluated for the treatment of saphenous vein grafts in the Symbiot 1 registry of 75 patients described at the ESC congress by G.J. Laarman, MD, of Amsterdam, the Netherlands. The Symbiot, which is now available in Europe, is comprised of a nitinol stent covered on both sides by 16-micron thick layers of ePTFE. In comparison with the WallStent, a non-covered stent also manufactured by Boston Scientific, major adverse coronary events were reduced by 61% based on the results from the registry. The SYMBIOT III trial is under way in the U.S. to generate data needed for marketing clearance. PTFE-covered stents also have been shown to be useful in the treatment of vessel perforations. Perforations have increased in interventional procedures in Europe over the past 10 years, due to more aggressive use of percutaneous intervention, resulting in a significant worsening of outcome.
As described by G. Stankovic, MD, of Milan, Italy, major adverse coronary events have been shown to occur at an eightfold higher rate (34.5% vs. 4.2%) for patients with a perforation, and rates for recurrence of a stenosis in the treated vessel or lesion are also considerably higher, by about a factor of two. While covered stents are not required to treat all cases of perforation, they provide a valuable option when other approaches, such as sealing of the lesion with a balloon, fail.
Approaches for improving the efficacy and cost-effectiveness of percutaneous intervention are also attracting investment. A number of companies are developing devices for detection of vulnerable plaque, potentially allowing lesion severity to be assessed more accurately than is possible with angiography, intravascular ultrasound, or non-invasive imaging methods. Cost savings could potentially result if plaque assessment indicated that a stent is not required, and savings will be even greater in the era of drug-eluting stents. Perhaps more importantly, vulnerable plaque detection devices could be used during PCI to assess other regions of the coronary vasculature (i.e., other than at the primary treatment site) that look suspicious on angiography, potentially allowing a developing lesion to be detected and treated that otherwise could cause a myocardial infarction even though the primary lesion remained patent. Since many heart attacks result from lesions that are not clinically significant on angiography, it is clear that improved methods to assess risk are needed.
Two companies, Epiphany and Volcano Therapeutics (Laguna Hills, California), exhibited products for vulnerable plaque detection at the ESC Congress. Epiphany is marketing a $500 thermography catheter in certain countries in Europe using a thermistor and pullback technique to map minute temperature gradients along the arterial wall, along with either a PC-based controller or a console to record and analyze the readings. The company also is developing a balloon catheter for use in stopping blood flow in the vessel during the measurement interval in order to achieve greater accuracy and sensitivity of vulnerable plaque detection. The device can be used to identify hot spots corresponding to lesions that are vulnerable to rupture in addition to the primary lesion being treated in an interventional procedure.
Volcano's thermography catheter uses a 5-thermistor basket catheter for pullback mapping. The target patient population is the approximately 50% of patients who have additional vulnerable lesions in addition to the primary lesion who suffer recurrent heart attacks after being treated. The catheter is being evaluated in two centers in Europe, with a third being added and plans to expand to as many as 30 centers by early 2003. Volcano has 30 patents on the use of thermography, and recently raised $3 million in venture and corporate funding from Domain Partners and Medtronic. Another company developing a catheter-based system for vulnerable plaque detection is Imetrx (Mountain View, California).
The introduction of new technologies and devices allowing expansion of the spectrum of applications of percutaneous intervention is expected to drive continued strong growth in the European market. As shown in Table 4, sales of coronary stent systems in Europe are projected to increase from about $422 million in 2001 to almost $1.5 billion by 2007, as a result of increased percutaneous interventional procedure volumes, a slight increase in the rate of use of stents in percutaneous coronary intervention (from a level of about 85% at present), and rapid growth in average selling price as drug-eluting stents are more widely adopted.
 |
Growing interest in cell transplants
A new area of opportunity is beginning to emerge in the European market for products for the treatment of cardiovascular disease that could potentially rival coronary stents in size, and that may result in the entry of a number of new suppliers. A number of companies are developing cell transplantation technologies with applications in the treatment of heart failure, and other applications may exist for tissue engineering of vascular grafts or angiogenesis for revascularization. As discussed by A. Zeiher of Frankfurt, Germany, at an ESC symposium sponsored by Aventis (Antony, France), very promising results have been observed in the TOPCARE study in which the use of stem cells isolated from bone marrow has been evaluated for restoration of cardiac tissue function. When injected into myocardium damaged by ischemia due to infarction, stem cells have been shown to differentiate and develop into cardiomyocytes, with the ability to enhance contractile function of the heart and provide recovery of myocardial viability. Treated patients exhibit a profound improvement in echocardiography parameters, ejection fraction and FDG-PET evaluations of myocardial function. Other tissue sources for stem cells that have been shown to be capable of differentiation into cardiomyocytes include fetal (embryonic) stem cells, neuronal stem cells, mesenchymal stem cells, skeletal muscle cells, and stem cells isolated from hepatic tissue.
Human embryonic stem cells are a particularly potent source, although a very controversial one. Studies by J. Itskovitz-Eldor of Haifa, Israel, described at the ESC congress, which have used one of the cell lines recently approved for use in the U.S., have shown that large numbers of cells can be propagated in vitro, and that the cells can subsequently be directed to develop along a number of different pathways via the use of different stimuli. When stimulated to develop into cardiomyocytes, the cells begin to express cardiac troponins and demonstrate contractile activity when electrically stimulated. By altering the stimulus, the cells can be directed to develop into smooth muscle cells (using platelet-derived growth factor), endothelial cells (using vascular endothelial growth factor), or hematopoetic stem cells if no stimulus is applied. However, controversy over the use of cells from human embryos has limited the pace of development. With the recent discovery of new sources for stem cells, the rate of progress in the field has accelerated significantly.
If cell transplantation therapy is developed to a clinically useful technique, it will address a large number of patients in Europe as well as worldwide. There are more than 20 million patients with congestive heart failure worldwide, including about 4.8 million in the U.S. There are about 500,000 heart failure patients in France alone, with an additional 120,000 new cases each year. Based on pricing for devices used for other types of cell-based therapy, the market for products used to treat heart failure could easily exceed $10 billion worldwide. Required technologies for stem cell therapy include devices for stem cell extraction and isolation, cell culture systems, and cell delivery systems. High-efficiency collection and culture systems will be key to successful development of stem cell therapy, since studies have shown that about 90% of the injected cells die within the first 24 hours. However, the surviving cells are functional, and can migrate within the heart to restore tissue viability over a wider region than the original implant zone. While at present researchers do not believe it will be feasible to completely reconstruct a new heart from stem cells, the most recent studies demonstrate that restoration of function to damaged regions of an existing heart is not only feasible but also durable, with some patients continuing to show improvement six months after treatment.
Some concerns about the therapy have arisen, particularly related to the possibility of arrhythmias resulting from failure of the implanted cells to contact in synchrony with the existing cells in the heart. Numerous studies have shown that newly formed cardiomyocytes can develop the ability to contract synchronously, at least in culture. In addition, some human studies involving small number of patients have not shown evidence of arrhythmia, including one reported at the ESC congress by Professor Tornaxz Siminiak of the department of cardiology at District Hospital (Poznan, Poland) in which skeletal myoblasts derived from thigh muscle were implanted in the hearts of 10 patients with heart failure. No arrhythmias were observed at up to eight weeks, although one patient died due to a myocardial infarction in an area distal to the original MI.
Miltenyi Biotec (Birgisch Gladbach, Germany) is one of a number of companies pursuing opportunities in stem cell therapy for cardiovascular disease. The company has developed a magnetic cell separation system that can be used to isolate stem cells based on their expression of specific cell surface markers such as CD133. Small magnetic beads coupled to anti-CD133 antibodies are mixed with extracted bone marrow, and the sample is then pumped through a magnetic separation chamber where the CD133-positive cells are retained. The cells can subsequently be eluted for use in an implantation procedure. Cost for the equipment is about EUR 30,000, and consumables used for cell selection cost between EUR 2,000 and EUR 3,000. A typical procedure takes between two and three hours.
Another exhibitor, Amaxa (Cologne, Germany), has developed a new gene transfer technology that may have applications in stem cell therapy. The company's Nucleofector technology uses electroporation to transfer DNA directly into a cell nucleus. While at present studies using cardiomyocytes are in the research stage, one potential application is to insert genes that would confer improved survival ability on cells prior to implant.
While cell transplantation has shown some promise, most researchers in the field are not yet ready to conclude that it will prove clinically useful in the long run. Many questions remain about the safety of the technique over the long term, including concerns about oncogenesis and continued reliability of contractile function. One study of 16 patients given autologous muscle cell implants described by P.C. Smits, MD, of Rotterdam, the Netherlands, found that one patient required implant of an ICD three months after treatment to maintain proper heart rhythm. The study used the Cordis NOGA catheter guidance technology to direct injection of cells into infracted tissue. However, other studies cited by Smits have shown much higher rates of arrhythmia, resulting in at least two deaths. As a result, Smits' team has decided to limit participation in further trials to only those patients having an ICD.
However, companies such as Cook Myosite, a joint venture between Guidant/Cook and the University of Pittsburgh (Pittsburgh, Pennsylvania); Geron (Menlo Park, California); Diacrin (Charlestown, Massachusetts); Advanced Tissue Sciences (La Jolla, California); Genzyme Biosurgery (Cambridge, Massachusetts) and Myosix (Paris) are continuing to develop improved techniques, and are moving forward with clinical studies. Genzyme Biosurgery and Myosix recently announced they will begin a global multicenter Phase I trial of autologous cell therapy to treat heart failure resulting from myocardial infarction, following the successful completion of a Phase I safety trial at Hopital Bichat (Paris).

