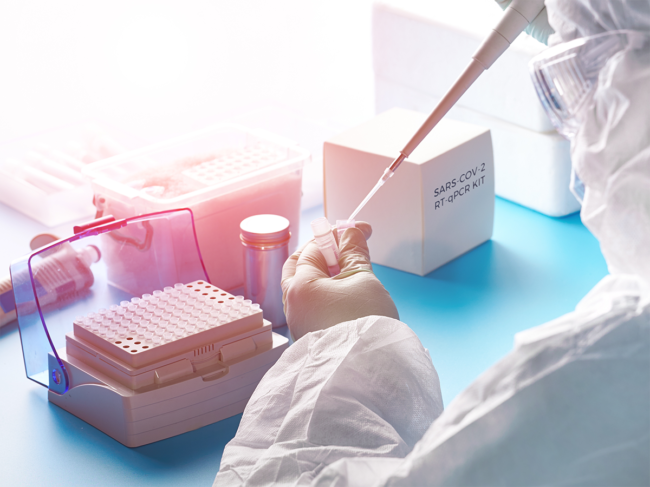
Coronavirus
Diagnosing the state of COVID-19 testing
Antibody testing for COVID-19 prepares for its closeup in bid to ease pandemic restrictions
Read MoreDiagnosing the state of COVID-19 testing
COVID-19 challenges med-tech regulators on traditional testing regimes
Read MoreDiagnosing the state of COVID-19 testing