The cost of developing and marketing drugs and devices in the U.S. is continuing to go up as the FDA raises its annual user fees for prescription drugs, biosimilars, generics and medical devices for fiscal 2016.
The increases, which go into effect Oct. 1, are based on numbers negotiated with industry as part of the five-year user fee agreements. For drug fees, the base number is adjusted for inflation and expected growth in the workload at the various offices in the agency's drug center.
The actual fees are derived by dividing the total amount the FDA can raise from each sector by the number of applications the agency expects to receive in the next fiscal year.
The device user fee agreement was negotiated a bit differently. Industry and the FDA agreed to the base fee for a premarket application (PMA) for each year of the agreement, which runs from fiscal 2013 through fiscal 2017.
All the other fees are a specified percentage of that PMA fee. This year, the device fees had to be adjusted a bit downward from what the agreement allowed because of statutory revenue caps and the number of applications expected, according to a notice in Monday's Federal Register.
The highest user fees are for prescription drugs and biosimilars. The 1.7 percent increase for new drug applications (NDAs) and biologic license applications (BLAs) involving clinical data for fiscal 2016 is relatively small compared with hikes in previous years. But at $2.374 million, the 2016 application fee is a hefty chunk of change, and it adds up to a 21 percent increase over the application fee of nearly $1.959 million set for fiscal 2013, the first year of the current PDUFA agreement.
The 2016 fee is based on the FDA receiving nearly 120 full NDAs and BLAs, the average of the number of full applications submitted over the past three years. Drugmakers submitting supplements or NDAs/BLAs not requiring clinical data will have to pay a $1.187 million fee.
In addition, drugmakers will be assessed a $585,200 establishment fee and a $114,450 product fee for each approved drug they have on the market. Altogether, the FDA plans to raise nearly $851.5 million in PDUFA fees. (See chart below.)
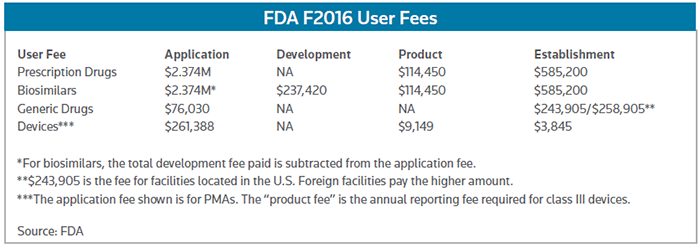
The biosimilar fees are the same as the PDUFA fees, except that biosimilar sponsors are on a "pay-as-you-go" scheme. Rather than paying the BLA fee all at once when they submit the application, they pay an annual biologic product development (BPD) fee until the application is submitted. Then, the amount of the application fee is reduced by how much they've already paid in BPDs. The BPD is set at 10 percent of the NDA/BLA fee each year, making it $237,450 for fiscal 2016.
The BDP payments begin when a biosimilar sponsor submits an investigational new drug application or within five days of the FDA granting an initial product development meeting – whichever comes first. The idea behind the unique fee structure was to help fund the FDA's costs of establishing the new pathway. (See BioWorld Today, June 10, 2011.)
While it works for the agency, it does have some disadvantages for the sponsor, which may have to begin paying the hefty fee years before the biosimilar is ready for approval. And should the sponsor back away from a candidate in development or the FDA refuses to accept the application, the sponsor is out all the BPD fees it's paid.
Generic drugmakers, on the other hand, pay their abbreviated NDA and supplement fees when they submit their application; nothing is due before then. Yet they can get a refund of 75 percent of their application fee if the FDA refuses to accept the ANDA or supplement.
That's a good thing, as they're in for a sizeable increase – nearly 30 percent – in ANDA filing fees in fiscal 2016. The FDA said the ANDA fees, along with fees for generic supplements and drug master files are going up based on a drop in the number of submissions in each of those categories this year. As a result, the ANDA fee will be $76,030, with supplements at half that amount.
The good news is that generic drugmakers will pay a little less in facility fees next year as the number of self-identifying plants making active pharmaceutical ingredients (API) or finished generic products for the U.S. market have increased. Thus, the domestic API facility fee is $40,867 for 2016; the foreign API facility fee is $55,867; the domestic finished drug facility fee is $243,905; and the foreign finished drug facility fee is $258,905.
When it comes to facility or establishment fees, device-makers have the best deal. In 2016, they'll be paying $3,845 in establishment fees, regardless of where they're based. The PMA fee will be $261,388 and the annual periodic reporting fee will be $9,149 next year. The device fee structure includes a number of other fees, as well as discounts for small businesses.

