Cardiovascular Device Update Contributing Editor
BARCELONA — The global market for cardiovascular devices is one of the most rapidly growing segments of the worldwide medical device market and is also one of the most dynamic. Segments of the market include devices for percutaneous coronary intervention (PCI), such as drug-eluting coronary stents (DES); devices used in heart rhythm management such as pacemakers and implantable cardioverter defibrillators (ICDs) and associated monitoring systems; heart valves; and certain niche devices such as left ventricular assist devices (LVADs), intravascular diagnostic and monitoring devices; and electrophysiology catheters. New developments in all the key segments of the market were discussed here at the World Congress of Cardiology (WCC 2006), a joint meeting of the World Heart Federation (Geneva, Switzerland) and the European Society of Cardiology (Sophia Antipolis, France).

The conference, which celebrated its 15th anniversary, attracted more than 30,000 attendees and was particularly noteworthy because of new challenges that emerged in the key DES segment, including a call for a comprehensive risk-benefit analysis to assess a possible elevated death rate in patients receiving DES implants. Other significant developments were described in technologies for management of patients with heart rhythm disorders, heart failure and heart valve disease.
Growth in the global cardiovascular device market is expected to average about 13% over the next five years, led by expansion in the market for ICDs, coronary stents, and percutaneously implantable heart valves. Growth is expected to be particularly strong in geographic segments outside of the major U.S. and European markets, including China, India and Eastern Europe.

Based on data from the most recent survey of cardiovascular diseases in Europe, the 2006 Euro Heart Survey published by the European Society of Cardiology, hospital discharge rates for cardiovascular diseases in Europe increased by about 50% between 1990 and 2004, while the rate for the European region increased by half as much. The hospital discharge rate for cardiovascular disease in the countries of the Commonwealth of Independent States (CIS) — which are 12 of 15 countries of the former USSR — is the highest of all the European regions included in the ESC survey at 30 per 1,000 inhabitants in 2004. That trend, coupled with the rapidly expanding economies of the CIS region, will combine to drive substantial growth in the emerging markets in Europe. Similar trends are likely to characterize emerging markets in Asia (particularly China) and Latin America.
Challenges for DES
The market for DES devices is one of the largest segments of the cardiovascular device market, estimated at $5 billion worldwide in 2005. The penetration of DES in the coronary stent market in the U.S. increased from 19.7% to 76.2% of percutaneous coronary intervention (PCI) procedures from the second quarter of 2003 to year-end 2004, according to data from the American College of Cardiology (ACC; Bethseda, Maryland), and has now reached 90%.
As shown in Table 2, penetration in Europe has grown from 5% of PCI procedures at the end of 2002 to about 55% at the end of 2005. Although there have been predictions that Europe and other regions will eventually follow trends in the U.S., adopting DES for all but a small percentage of patients, new information presented by Edoardo Camenzind, MD, of University Hospital (Geneva, Switzerland) raised questions about the safety of first-generation devices that could slow or reverse adoption trends. Camenzind, along with William Wijns of the OLV Hospital (Aalst, Belgium) and Gabriel Steg, MD, of Groupe Hospitalier Bichat-Claude-Bernard (Paris) presented the results of a meta-analysis of results of clinical trials of the first-generation DES devices, including the Cypher stent from Cordis (Miami Lakes, Florida) and the Taxus stent manufactured by Boston Scientific (Natick, Massachusetts). The meta-analysis combined results from all available published or presented randomized trials of first-generation Cypher and Taxus drug-eluting coronary stents, analyzing adverse event rates in the pooled patient population included in the trials.

Four trials (RAVEL, SIRIUS, E-SIRIUS and C-SIRIUS) were included which used Cypher, and five were included (TAXUS I, II, IV, V and VI) that used Taxus. The most recent available outcome data were included for all trials. The key finding was a higher incidence of death and Q-wave myocardial infarction in patients with DES at three years. The difference was statistically significant for Cypher at 6% in the DES patients vs. 4% in controls.
In the Taxus trials, a higher rate of death and MI was also found at three of four time points analyzed, but the difference was not statistically significant (3.5% vs. 3.1% for controls at three year follow-up). The additional cases of death and MI were considered to be surrogates for stent thrombosis, indicating that the investigators believed that to be the primary cause of overall mortality and MI for the DES patients.
Another analysis, conducted by Alain Nordmann, Matthias Briel and Heiner Bucher of the Basel Institute for Clinical Epidemiology (Basel, Switzerland) reached a similar conclusion — that is, a higher rate of overall mortality for patients with DES implants. The difference in death rate was primarily due to a higher rate of non-cardiac mortality in the group receiving Cypher stents, with a statistically significant odds ratio of 1.65 (p = 0.08) for the Cypher group at four years, based on the results of two trials. An even higher odds ratio of 2.74 was found at two years in the Cypher group.
While the Taxus patients did not have a statistically significant increase in non-cardiac mortality, there was a non-significant trend toward increased mortality at all time points analyzed. An analysis of the non-cardiac deaths in the Cypher group showed that 15 of the 35 non-cardiac deaths were attributable to cancers of a variety of types. The next most common cause of non-cardiac death was stroke.
None of the trials included in the meta-analysis were powered to detect a difference in non-cardiac death. However, the investigators involved in both meta-analyses concluded that there is evidence of excess adverse events, including death, in patients receiving DES implants vs. bare metal stents.
DES now over-used?
In a commentary on the Camenzind presentation, Salim Yussuf of McMaster University (Hamilton, Ontario) called for Euro Heart Surveys to be performed to determine the need for drug-eluting coronary stent therapy, stating that DES technology is over-used, particularly in patients with stable, non-life-threatening coronary artery disease.
Published guidelines, such as those from the ACC, state that there is Class One (Level A) evidence, the highest level designated, for use of DES as an alternative to bare metal stents for subsets of patients in whom clinical trial data suggest efficacy.
Yussuf said that he believes DES use should be limited to 40%-50% of PCI procedures, rather than the 80% level now prevailing worldwide, and that their use should be limited to patients with MI, high-risk unstable angina, and cardiogenic shock. Camenzind, however, emphasized in his presentation that the results applied only to the two first-generation drug-eluting stents.
The European experience
There are now a number of DES devices on the market in Europe, as shown in Table 3. Based on the results of a 2005 survey of a database, including more than 13,000 patients treated in 134 centers in 39 countries in Europe, the devices are used in 10%-80% of PCI procedures in Europe, depending on the country. Stents of any type (either DES or bare metal) are used in 93.5% of PCI procedures. The highest rates of utilization of PCI are in the central European countries (36.6 patients per million inhabitants), followed by the western European countries (27.8/million), the Mediterranean countries (21.5/million), and the countries in northern Europe (7/million). About 68% of stent procedures performed in Europe use a single stent, 19.5% use two stents, and 7% use three or more stents. Bare metal stents are the most widely used stent type in central Europe, while DES devices are the most widely used in the Mediterranean countries.

New stents continue to be introduced in Europe that could address some of the drawbacks of first-generation devices and help to drive continued market growth. For example, results of pivotal clinical trials with the Abbott Xience stent were presented at the WCC congress by Patrick Serruys, MD, PhD, of Rotterdam, the Netherlands. Six-month follow-up data from the SPIRIT II trial of Xience, which involved a randomized comparison to the Taxus stent, showed a three-fold lower late loss of restenosis (0.11mm for Xience vs. 0.36mm for Taxus) as well as a significantly lower rate of restenosis (1.3% vs. 3.5%) in a patient group having relatively uncomplicated (A, B1 and B2) lesions.
Although the number of patients was small (152), an encouraging finding is a low rate of late thrombosis of 0.5% vs. 1.3% for Taxus, and a target lesion revascularization rate of 2.7% vs. 6.5% for Taxus. The data indicate that advanced-generation DES devices, using different drugs and polymers, may enable reduced rates of adverse events to be realized, helping to negate at least some of the disadvantages of current-generation drug-eluting stents compared to bare metal stents.
If a link between long-term development of cancer and first-generation DES devices is borne out in larger studies, use of one of the newer drugs, or perhaps elimination of drugs altogether by use of bioactive stents, is a possible solution.
Coming: next-generation DES
Next-generation stents now on the market in Europe include the Cypher Select Plus from Cordis, which includes a hydrophilic coating at the distal end to provide improved deliverability and crossability. The crossing force for the Select Plus has been reduced by 94% compared to the first-generation Cypher. Another next-generation design that could avoid issues of long-term adverse events associated with existing drugs and polymers is the bioengineered Genous R-Stent from Orbus Neich (Hong Kong). The Genous employs a surface coating of CD34 monoclonal antibodies to attract endothelial progenitor cells from the blood, promoting vascular healing and development of a biocompatible, non-thrombogenic tissue layer covering the stent.
Eighteen-month results from the HEALING II trial, presented in May 2006, have shown a moderate rate (6.3%) of target lesion revascularization (TLR) at 6, 9 and 18 months, with the rate stable after six months. In addition, late luminal loss was shown to regress by 18%, which has not been shown to occur with drug-eluting stents, and is observed to a much smaller degree with bare metal stents. The somewhat high rate of TLR indicates that promotion of endothelial coverage of the stent may not be as effective in eliminating restenosis as elution of drugs that inhibit cell proliferation, but on the other hand more effective healing may occur, leading to reduced long-term adverse events.
Another bioactive stent designed to promote endothelial cell coverage is being developed by Guidant/Boston Scientific (Natick, Massachusetts), as described by R. Blindt, MD of University Hospital (Aachen, Germany) at the WCC meeting. Blindt had evaluated a new stent that uses the Tetra stent platform from Guidant with a polymer coating loaded with cyclic integrin-binding RGD (cRGD) peptide in animal studies. The cRGD-polymer coating was shown to promote endothelialization of the stent, resulting in a significant reduction in neointimal area and stenosis at 12 weeks compared to bare metal stents or stents coated only with the polymer.
Alternatives to DES
Another possible approach to prevention of long-term adverse effects of polymer-coated DES is the use of bioabsorbable materials, including completely bioabsorbable stents, which would eliminate the presence of a permanently implanted foreign body, in principle allowing restoration of a normal artery.
Biotronik (Berlin) is developing a magnesium stent, the Absorbable Metal Stent, which has proven safe in a first-in-main study, the PROGRESS-AMS trial. However, the restenosis rate at four months was excessive and not in line with clinical standards, indicating that some adjustment of the design is needed, including adjustment of the stent degradation rate, to achieve optimal preservation of long-term patency. Biotronik is also evaluating drugs that could be combined with the absorbable stent platform to address the restenosis issue.
As shown in Table 3, other stent suppliers have developed devices with bioabsorbable polymers, in an attempt to avoid the long-term adverse effects of permanent polymers. However, as discussed by Bernhard Meier, MD, of University Hospital Bern (Bern, Switzerland) at a Cordis-sponsored symposium on DES held during the WCC meeting, potential issues with bioabsorbable stents include long-term restenosis, inflammation or restenosis due to the breakdown products of the absorbable materials, difficult delivery characteristics, embolization of fragments and the possibility for cells to attach to the stent fragments.
To come, longer-term trials
The coronary stent market in Europe and worldwide continues to present a significant opportunity for suppliers. However, market growth may be impacted by the safety issues raised at the WCC meeting, even though definitive data from large-scale trials is not yet available.
A mini-poll taken by the ESC at the WCC meeting found that none of the four physicians interviewed would choose a DES device rather than a bare metal stent given the results announced at the congress. However, the results provide manufacturers with key new information on the drawbacks of first-generation devices, and can help in directing development of next-generation stents with improved properties. In addition, technologies that can help to more precisely determine which patients will benefit from drug-eluting stents are likely to become more important in the marketplace.
For example, data from the Viability-guided Angioplasty after acute Myocardial Infarction (VIAMI) trial, presented at the WCC meeting by Gerrit Veen, MD, of University Hospital VU (Amsterdam, the Netherlands), shows that patients who have viable tissue in the infarct zone following MI and thrombolysis benefit from PCI and stenting, whereas those not showing viability have no net benefit. Viability in the study was determined using low-dose dobutamine echocardiography.
Technologies to assess the risk of restenosis and thrombosis could also become more important in allowing physicians to improve the selection of patients for DES implants. Patients who are likely to discontinue dual anti-platelet therapy, those with low left ventricular ejection fraction, patients with bifurcation lesions, and patients with renal failure or diabetes are all at increased risk for late thrombosis, which is the adverse event of most concern to cardiologists.
Another approach discussed at the WCC meeting by T. Lefevre of Cardiologie Interventionnelle (Massy, France) is use of the SYNTAX scoring system, developed in conjunction with the SYNTAX trial of the Taxus DES. The scoring system is based on the EuroScore method for risk assessment, and is being used in the trial to determine which patients will be revascularized using stents vs. bypass surgery.
Percutaneous heart valve technology
Another segment of the interventional cardiology market addressed at the WCC conference that is poised to emerge as a significant opportunity for suppliers is devices for percutaneous treatment of heart valve disorders. As shown in Table 4, about 300,000 patients are treated annually worldwide for heart valve disorders using open heart surgery techniques.

A new development in the field is the use of minimally invasive technologies for heart valve repair and replacement. At present, the primary less-invasive techniques employed include use of mini-sternotomy, video-assisted endoscopic surgery, video-directed endoscopic surgery, and robotic surgery. According to Friedrich Mohr, MD, PhD, of the University of Leipzig (Leipzig, Germany), who discussed minimally invasive heart valve therapy at the WCC meeting, the use of minimally invasive technologies in Europe is growing, with more than 6,600 patients treated, based on a recent survey of 10 centers performing minimally invasive surgery. At Mohr’s institution, the number of patients treated with minimally invasive surgery for mitral valve disease outnumbers those treated with conventional open surgery by five to one.
The latest trend in Europe is the development of percutaneous device therapy for heart valve disorders. At present, a significant number of mitral valve patients are treated using percutaneous balloon valvuloplasty, primarily using the Inoue double-balloon catheter from Toray Medical (Tokyo). About 34% of patients with mitral stenosis are treated with balloon valvuloplasty, based on data from the most recent Euro Heart Survey. However, a new segment of the market is about to emerge for devices used in other types of percutaneous heart valve procedures, including repair and replacement of both aortic and mitral valves.
At present, devices for percutaneous treatment of valve disease are experimental and are used in clinical trials to treat primarily the one-third of patients with severe valve disease who are not candidates for surgical therapy. However, in analogy to the market for coronary stents, it is likely that there would be strong demand for percutaneous treatment if safe and effective devices become available. Many patients with heart valve disease are not treated at present because their condition is not sufficiently serious to warrant the risk of surgery. Such patients may be candidates for less invasive percutaneous therapy. For example, in the U.S. the number of patients with mitral valve regurgitation outnumbers those who are treated surgically by five to one.
Product variety
According to Mohr, there are now 27 different devices under development for percutaneous treatment of heart valve disorders. As discussed by Alec Vahanian, MD, of Bichat Hospital (Paris) at the WCC sessions, two devices are now in advanced stages of development for mitral repair, including the MitraClip from EValve (Menlo Park, California) used to perform minimally invasive repair via the Alfieri technique; and the Mobius Leaflet Repair System from Edwards LifeSciences (Irvine, Calilfornia), the leading supplier of heart valves worldwide.
Encouraging results have been achieved so far using the EValve MitraClip, with 80% event-free survival at 24 month follow-up in patients with acute procedural success in the EVEREST II trial. A percutaneous device for performing mitral annuloplasty, the Viking system from Edwards LifeSciences, is also under development. The Viking system consists of proximal and distal self-expanding stents that serve as anchors and a spring-like bridge that foreshortens over a period of about six weeks to complete the annuloplasty.
In initial clinical studies with the Viking, some recurrent mitral regurgitation was observed, but that could be due to the delay needed for the device’s bridge to set. Vahanian said that the primary indications for use of the Viking device include functional mitral regurgitation, since surgical treatment is risky and can have poor results; degenerative mitral regurgitation; and cases of moderate regurgitation where the device could be used to delay surgery. However, questions remain for all of the devices regarding limitations on subsequent open surgery in patients receiving an implant, as well as the efficacy and cost-effectiveness of the device compared to other alternatives such as medical therapy or cardiac resynchronization therapy. With regard to compromising subsequent surgical treatment, Vahanian noted that surgeons had been able to remove mitral clips in five of six patients who had implants and perform successful surgical treatment.
Another device under development for percutaneous treatment of mitral valve disorders is the Mitralign system, a catheter-based device that deploys a series of implants along the perimeter of the mitral valve and then cinches the implants together to reduce the valve diameter. The device is under development by Mitralign (Tewksbury, Massachusetts). Yet another device, the Percutaneous Septal Sinal Shortening or PS3 system, is being developed by Ample Medical (Foster City, California). The PS3 system employs a bridge element connected to anchors which are placed in the interatrial septal wall and the great cardiac vein. The bridge is then progressively shortened to reduce the diameter of the mitral valve annulus, eliminating regurgitation. Initial studies in sheep reported earlier this year have demonstrated successful and durable treatment of mitral regurgitation, and initial human implants have been performed in a clinical study initiated in Caracas, Venezuela.
Targeting aortic repair
Advances in percutaneous aortic valve repair and replacement were also described at the WCC sessions. Aortic repair has attracted significant development interestt since aortic valve stenosis accounts for 25% of all valve disease worldwide, and is the most frequently encountered type of valve disease in Western countries, affecting 2%-3% of the population over the age of 65. Without treatment, only 20% of patients survive three years after diagnosis, whereas with surgical treatment the survival rate jumps to 90%. However, 32% of patients with severe valve disease are not referred for surgery due to pre-existing cardiac or non-cardiac disease.
In some cases, patients are not treated solely because of their advanced age. Companies involved in percutaneous aortic valve development estimate that as many as three million people in the U.S. have aortic valve stenosis, but only a small percentage are treated. Alain Cribier, MD, of Rouen University Hospital, (France), an expert on percutaneous heart valve therapy, is collaborating with Edwards LifeSciences in clinical trials of the Cribier-Edwards percutaneous aortic valve. Cribier described the current status of the Edwards development program, which now includes more than 200 patients treated worldwide. The clinical trial program has proceeded through a number of phases as new versions of the valve and delivery system have been developed.
At present, studies are underway employing the newly designed FLEX Catheter that facilitates crossing of tortuous anatomy with the device, allowing a retrograde approach to be used. Another advance is the use of a larger 26 mm diameter stent framework, which has eliminated a problem with perivalvular leaks. Trials are underway in the U.S. (the REVIVAL trial) and Europe (the REVIVE trial). Cribier expects the retrograde approach using the FLEX catheter to be the primary technique used to deploy the valve, with a transapical or antegrade approach reserved for only a limited number of cases.
. . . and still more
Other percutaneous heart valve devices under development include the ReValving system from CoreValve (Irvine, California); the Lotus valve from Sadra Medical (Campbell, California); the PTMA device from Viacor (Wilmington, Massachusetts); the Carillon system from Cardiac Dimensions (Kirkland, Washington); and the Coapsys system from Myocor (Maple Grove, Minnesota). Other companies involved in percutaneous heart valve development include AorTx (Palo Alto, California), Medtronic, Cook (Bloomington, Indiana), Heart Leaflet Technologies (Roseville, Minnesota), MitralSolutions (Fort Lauderdale, Florida), Direct Flow Medical (Santa Rosa, California), 3F Therapeutics (Lake Forest, California), ValveXchange (Los Angeles, California) and QuantumCor (Lake Forest, Callifornia), which is developing the Q-Care catheter system employing RF energy to thermally reshape heart valve tissue. CoreValve has one of the more advanced development efforts, having completed a first-in-man trial with its PAVR percutaneous aortic valve and demonstrating feasibility as well as hemodynamic improvement. The CoreValve device consists of a self-expanding nitinol frame and a porcine pericardium valve.
Recent improvements in the design include development of an 18 Fr version, facilitating percutaneous access compared to the 22 Fr minimum diameter for the Cribier-Edwards valve. So far, more than 70 patients have received implants of the CoreValve device in clinical trials.
Valve construction: nanosynthesis
At a symposium sponsored by CoreValve held as part of the WCC meeting, Patrick Serruys, MD, PhD, described another new valve design, the PercValve, which is being developed at Erasmus Medical Center (Rotterdam, the Netherlands). The valve is fabricated using metal nanosynthesis technology, which involves vacuum deposition of metal to form complex mechanical structures. The valve consists of eNitinol microporous leaflets and a metal framework. A key feature is use of a 10 Fr catheter for implantation of the aortic version of the PercValve; another version uses a 4.8 Fr catheter.
The goal, as described by Serruys, is to develop devices that are truly minimally invasive, allowing treatment of the large reservoir of patients with valve disease who are not at present referred for surgery. Serruys noted that a recent study has shown that 37% of patients with asymptomatic valve disease become symptomatic on exercise testing, and that asymptomatic patients typically undergo progressive deterioration. Such patients could potentially benefit from valve repair or replacement if an appropriate minimally invasive technology becomes available.
Heart rhythm therapy expands
Another major topic at the WCC sessions was device-based treatment for heart rhythm disorders, including pacemaker and implantable cardioverter defibrillator (ICD) therapy. The combined worldwide market for pacemakers, ICDs and related equipment in 2006 is estimated at in excess of $10 billion by suppliers, including $6.4-$6.7 billion for ICDs and $3.6-$3.7 billion for pacemakers, with growth of the combined market projected at 8-12%.
The market for ICDs slowed somewhat in 2005 due to recalls of some devices in the U.S., but suppliers are predicting a rebound in 2006. An important trend in the market is the addition of a wide range of monitoring features to the devices, including sensors for tracking physiological parameters such as blood pressure and fluid status, as well as monitoring electrocardiograms and device parameters. That trend is transforming the competitive structure of the market, from one focused on device features, reliability, and pricing to a broader spectrum involving disease management.
With automatic remote monitoring systems, physicians can not only track device performance to detect impending problems and need for adjustment, but can also monitor patient status for early indications of decompensation in heart failure, dangerous heart rhythms, or adverse trends in cardiovascular status.
Device monitoring
Device monitoring also addresses an issue that has arisen as a result of the success of ICDs in penetrating the global market, namely the dramatic increase in the number of patients with implantable devices who must be monitored routinely. The average physician is now monitoring more than 40 ICD patients routinely, creating a data overload if the physician is forced to analyze the complete range of data reported on a regular basis. Monitoring systems can automate the process, sending data only when necessary, and can increase patient safety by providing immediate early warning if the patient’s condition deteriorates.
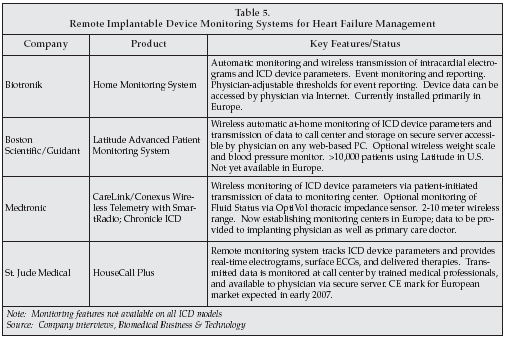
However, while such systems have been widely deployed in the U.S., deployment in Europe is just beginning, at least for U.S.-based manufacturers, due to differing requirements for data security and privacy in Europe as well as issues with allocation of radiofrequency bands for telemetry. Medtronic, the leading supplier, is just now beginning to set up monitoring centers in Europe, and will establish a dedicated server for its CareLink system that is only accessible by physicians.The company believes that monitoring will rapidly become a key competitive factor in the market in Europe.
Likewise, Boston Scientific/Guidant sees remote monitoring as a key element of future products in the heart rhythm management sector. Biotronik has been providing its Home Monitoring system to patients in Europe at no added charge for some time, allowing every patient to be tested every day. St. Jude and Guidant plan to expand deployment of their monitoring systems from the U.S. to Europe, with St. Jude expecting a CE mark early next year.
Monitoring goes implantable
The benefits of implantable monitors in heart failure were described at the WCC meeting by Lars Ryden, MD of Karolinska University Hospital (Stockholm). In a Phase 1 study using the Medtronic Chronicle IHM device, a reduction in hospitalizations from 1.08 per patient-year to 0.47 was observed, attributable to use of monitoring with the Chronicle. In the COMPASS-HF study, there was a statistically significant 41% reduction in combined heart failure-related hospitalizations, emergency department, and urgent care visits in a group of NYHA Class III patients whose physicians had regular access to the Chronicle data compared to controls without data access.
A new trial is now planned in Europe starting in 2007 that will be similar to the Reducing Decompensation Events Utilizing IntraCardiac Pressures in Patients with Chronic HF (REDUCE-HF) trial now underway in the U.S. using the Chronicle-ICD. Ryden believes implantable monitors will provide a new approach to heart failure management, and will have applications in other areas such as management of patients on hemodialysis.
A key feature of the technology is its ability to provide improved autonomy and quality of life for heart failure patients. Implantable monitors are one example of e-health technology that is now beginning to be used to improve outcomes and reduce costs for a number of chronic disease patients.
Other examples are non-invasive telemonitoring systems such as the AlereNet system marketed by Alere Medical (Reno, Nevada), discussed at the WCC meeting by Barbara Lamp of the Heart and Diabetes Center (North Rhine-Westphalia, Bad Oeynhausen, Germany), and the HomMed unit of Honeywell (Minneapolis). Lamp cited projections that e-health will account for 5% of total health expenditures in Europe by 2010.

