While Congress edged closer to a deal that would put the government back to work, the impact of the shutdown is piling up at the FDA even though it had carryover user fees to keep many of its activities moving for the past few weeks.
With more than half of its work force remaining on the job during the partial shutdown, the FDA continued reviewing supplements and new drug and biologic license applications (NDAs/BLAs) submitted before Oct. 1. But its inability to accept most applications since then is likely to create a bottleneck once Congress agrees on a spending bill to fully reopen the government. (See BioWorld Today, Oct. 3, 2013, and Oct. 9, 2013.)
When it’s fully staffed again, the FDA will have to process all the fiscal 2014 establishment and product fees that were due Oct. 1, while tending to the drug and biologic applications and sponsor requests for meetings that have been idling. (See BioWorld Today, Aug. 2, 2013.)
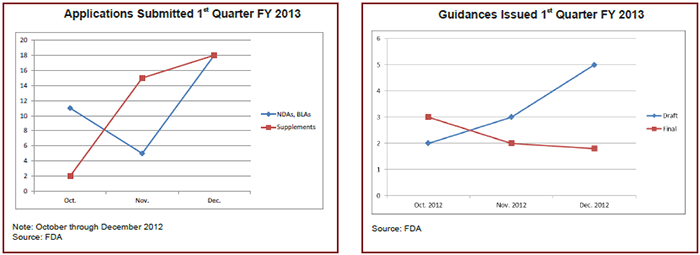
Since the number of NDAs/BLAs can vary greatly from year to year and month to month, it’s hard to say how many applications are waiting for the FDA to return to business as usual. A look back at last year shows that, in October 2012, the agency received two supplements and 11 NDAs/BLAs, four of which were for new molecular entities. (See chart below.)
If the agency gets hit with a lot of applications and requests, it could cause a bottleneck for a few months and delay review times.
Stalled Guidance
Drug applications won’t be the only traffic jam at the FDA. A few months ago, the agency’s drug center released an ambitious list of 63 draft guidances it planned to issue by the end of the year.
The list included guidance on submitting clinical pharmacology data as evidence of biosimilarity, using the Animal Rule to develop medical countermeasures and developing drugs to treat Alzheimer’s, pulmonary tuberculosis, chronic hepatitis C infections, community-acquired bacterial pneumonia and antibacterial therapies for diseases with limited or no alternative treatments. (See BioWorld Today, July 30, 2013.)
Other guidances in the works dealt with clinical trial endpoints, electronic submissions, and risk evaluation and mitigation strategies. While a few of the drafts made it out the door before the shutdown, most of them were stalled.
In the last three months of 2012, the Center for Drug Evaluation and Review (CDER) issued 10 draft and 10 final guidances, according to the FDA. How many CDER will issue yet this quarter depends on how far along the documents were before the lapse in appropriations. (See chart below.)
Another traffic block impacting some of the FDA’s work is the White House Office of Management and Budget (OMB), which has to approve all agency requests for information collections and extensions of those collections.
The information collected can involve guidance, applications, research, postmarket surveillance, regulations, administrative policies, etc.
For instance, the FDA requested extensions last month of several existing information collections that are set to expire Oct. 31 , including such things as postmarketing reports, requirements for submitting NDAs and BLAs in electronic format and guidance on hypertension drug labeling for cardiovascular outcomes.
The FDA requests are just a fraction of the more than 800 information collection requests under review at OMB’s Office of Information and Regulatory Affairs, which was shut down during the lapse in appropriations.
Inspections Delayed
Routine inspections of drug facilities, which also were halted during the shutdown, could create yet another traffic jam for the FDA. It’s hard to say how many routine inspections the agency would have done in the U.S. this month as it doesn’t track those numbers on its performance dashboard. But it does track the number of routine inspections its international offices conduct.
From October 2012 to March 2013, an average of three routine inspections a month were conducted at drug facilities in other countries, but the actual number of inspections per month ranged from one to six.
Meanwhile, the FDA’s Office of Prescription Drug Promotion has indefinitely postponed its third-quarter enforcement webinar originally slated for Oct. 28 because of the shutdown.
And elsewhere, the Patent and Trademark Office, which has remained open during the appropriations lapse, has pushed its Oct. 30 public meeting on copyright policy issues back to Dec. 12, citing complications arising from the government closure.
