HONG KONG & NEW DELHI – Middle East respiratory syndrome (MERS) is in the spotlight across Asia, as researchers search for drugs to fight a virus many fear could be as devastating as SARS was in 2003.
A group of Chinese researchers has made progress developing the country's first anti-MERS drug while the National Health and Family Planning Commission (NHFPC) launched on June 12 a series of prevention and control plans. The NHFPC released on Friday a technical guide, a diagnosis and treatment scheme and a list of MERS experts along with the notice to raise more attention to the control and prevention of the disease.
China confirmed its first and only-to-date imported MERS case on May 29, after being alerted of a potential outbreak by neighbor South Korea.
"Research and development institutes are making efforts to develop MERS vaccines," said Mao Qunan, the head of the NHFPC's press and publicity department. "If there's more information on the variation, it will have fundamental impact on the development of MERS vaccines and anti-MERS drugs."
"Chinese researchers have been studying the characteristics, transmission capacity, transmission channels of the MERS virus . . . the experts have reached consensus in some areas, but there're still debate over other results," said Mao. "From what we can see now, the transmission capacity of MERS is still limited, but the similarities and differences between SARS and MERS are still to be further studied."
Chinese researchers led by Jiang Shibo from Fudan University's School of Basic Medicine have been searching for ways to prevent and treat MERS before the breakout this year.
"MERS is only 70 to 80 percent similar to SARS," Lu Lu, associate professor and one of the researchers on Jiang's team at Fudan University, told BioWorld Asia. "Because of the differences in transmission channels and genome, MERS is not as close to SARS as some people would think even though their symptoms are very alike. The breakout in South Korea is probably caused by the insufficient precaution. MERS is not as easily transmitted as SARS but this could change if there were to be variations."
CHINESE TEAM'S CANDIDATE WORKS IN ANIMALS
Jiang's team recently found a new polypeptide HR2P-M2 is effective in fighting MERS. The group successfully tested HR2P-M2 in animals and found that the polypeptide can be used through the nasal cavity in the form of a mist spray.
"The polypeptide has two features: It is a specific drug that only targets MERS-CoV and the drug works at the virus invasion stage to stop the fusion of S2 protein," said Lu.
"We've seen very positive results in animals for the prevention of MERS," Lu added.
This is the first anti-MERS drug developed by Chinese scientists and the team is planning to publish its results in an international infectious disease journal.
"Besides the anti-MERS polypeptide, we're also working on antibody and vaccine projects with U.S. researchers," said Lu.
To date, however, few drug companies are rushing to develop or commercialize anti-MERS products in China.
"I haven't seen any pharmaceutical companies here start making MERS drugs," said Li Yanzhao, head of the molecular diagnosis department at Beijing Kinghawk Pharmaceutical Co. Ltd., which is among the first developers of MERS-dedicated diagnostics.
"Not a lot pharmaceutical companies are willing to work on commercializing the drugs because the patient pool is small and it's not a particularly profitable area because many infectious diseases such as SARS and Ebola come and go after a relatively short period of time," said Lu. "One company contacted us and wants to make a nasal spray version of the drug. . . . But the collaboration is still in discussion."
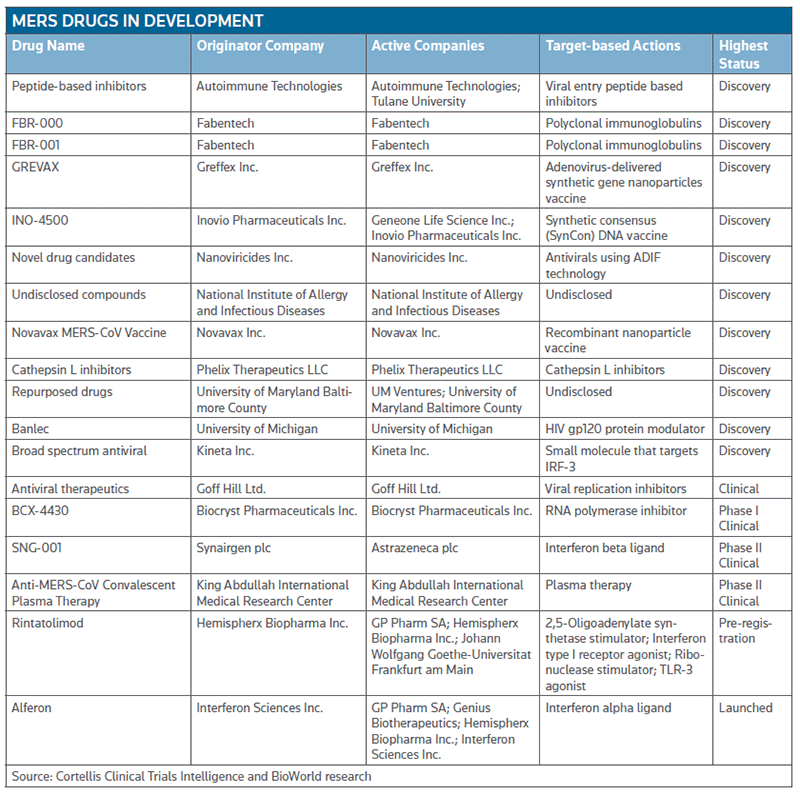
Companies around the globe are throwing everything they have at MERS. According to Cortellis Clinical Trials Intelligence and BioWorld research, 13 candidates are in the discovery stage (most described as antivirals), three are in clinical trials and two interferon-based products are being suggested for use in MERS cases. (See the table below.)
FIRST, WE DIAGNOSE
However, Chinese companies have been developing MERS-related diagnostic tools for regular disease prevention purposes before the outbreak in South Korea.
"We started making MERS diagnostic kits in the latter half of 2014 for reserve purposes," said Li. "This year we have orders from Beijing, Shanghai and Guangzhou not because of outbreak fears but as a part of the national reserve strategy.
"There's not a rise in the need for MERS detecting reagents because the situation in China is not as intense as in South Korea and the control measures are well practiced," said Li. "The antibody developed to fight against diseases such as MERS is also registered as a medical device unless it's in the form of injection or oral solution."
Among East Asian countries, South Korea has been most affected by the viral disease that surfaced in 2012 in the Middle East but did not make its way to the region until last month.
THE LATEST COUNTS
As of June 12, South Korea's Ministry of Health and Welfare had recorded some 126 cases of MERS and 11 deaths of people who tested positive for the virus. No other country has recorded deaths or even significant outbreaks but both Hong Kong and Mainland China are on high alert. In the single case reported in Mainland China, the patient traveled to the country from South Korea via Hong Kong.
The World Health Organization and the Republic of Korea's Ministry of Health and Welfare are working together to gain information and review the situation in Korea including the epidemiological pattern, the characteristic of the virus and clinical features. The team also will assess the public health response efforts and provide recommendations for response measures going forward.
The outbreak in South Korea has once again highlighted the importance of developing new drugs and vaccines for viral diseases that are typically put on the backburner over the last decade.
The coronavirus that causes MERS was believed to have been contained in the Middle East, with no threats of its spread elsewhere, until it surfaced in South Korea.
The outbreak had a visible impact. It happened, for example, just ahead of the World Conference of Science Journalists (WCSJ) in Seoul this month and some participants cancelled their visits while far fewer people ventured out onto the normally bustling streets of Seoul, most with surgical masks on their faces. Hundreds of schools have been closed.
The scenes were reminiscent of the high days of the SARS outbreak in Hong Kong and China, when fear of the disease emptied the streets.
In Seoul last week, Hong Kee-Jong, director of the Institute Pasteur Korea, held a session on MERS at the WCSJ and said health authorities were confident that the outbreak would be contained soon, given the country's advanced labs and hospitals.
Sophisticated facilities notwithstanding, the case of one infected patient who waited in an emergency ward for 2.5 days likely led to the spread of the outbreak and may have been the cause of 55 other infections.
A referral system of hospital admissions and a practice of relatives to look after patients while in hospital that is common in Asia add to the risk of transmission. Most of the infections in hospitals and health care facilities were due to sharing of rooms by patients or their relatives, and of medical instruments, said Hong.
The good news is that South Korea appears well equipped to deal with the outbreak. Compared to the 30 percent to 40 percent fatality rates seen in the outbreak in the Middle East in 2012, South Korea has witnessed 10 percent fatality rates.
Preliminary analysis of two viral genetic sequences, one released by Korea's health ministry and the second by China's Center for Disease Control and Prevention, showed that the new MERS sequences are not very different from the ones in the Arabian Peninsula and "are unlikely to present different virulence or transmission properties." They show only minor changes or mutations.