BB&T Contributing Editor
DÜSSELDORF, Germany — The worldwide markets for products used in point-of-care (POC) testing and for monitoring of vital signs in patients outside the traditional hospital setting have been growing at rates in excess of those for the overall medical device and diagnostics market. Among the segments of the market exhibiting the most rapid growth are products for whole blood glucose testing, POC coagulation testing products, POC cardiac markers, and patient monitoring products used for home and ambulatory monitoring.
The European market is no exception, although POC testing and remote patient monitoring have advanced less rapidly in Europe than in the U.S. The 2006 MEDICA exhibition, held here in mid-November, provided an opportunity to view the latest developments in POC testing and patient monitoring, as well as advances in many other segments of the medical device market, including diagnostic imaging, surgical navigation, endoscopy, drug delivery and electrostimulation therapy.
Although held in Europe, the MEDICA exhibition attracts vendors from all the major world markets, including suppliers from emerging markets such as China, India, and Latin America, which represent some of the most rapidly growing segments of the global market. In addition, suppliers from emerging markets are having an impact on markets in the developed regions by offering products with competitive features at low prices. Chinese companies in particular are beginning to expand outside of their domestic market to target penetration of the European and U.S. markets, with product offerings that in some cases offer features equivalent to those provided by established suppliers.
POC testing expanding in Europe
The market for in vitro diagnostic POC testing products in Europe is one segment that is attracting growing interest among suppliers, from both from developed as well as emerging countries. As shown in Table 1, the total market for POC testing products in Europe is estimated at more than $3.3 billion for 2005, and the market is forecast to more than double by 2011, approaching $6.9 billion.
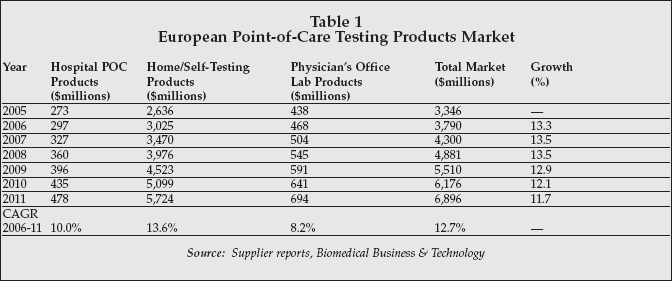 |
Products for home and self-testing, primarily whole blood glucose monitoring products, are expected to exhibit the most rapid growth, followed by hospital POC testing products and physician's office laboratory products. Other important products within the home and self-testing segment include coagulation self-testing products, as well as home pregnancy and fertility test kits.
Testing whole blood glucose
The market for whole blood glucose testing products, estimated at almost $2.9 billion in Europe in 2006, is being driven by continued increases in diabetes prevalence worldwide as well as by growing appreciation of the benefits of improved management of blood glucose levels on long-term outcome. Tight glucose control (TGC) programs in hospitals are an important factor, with some suppliers of hospital bedside whole blood glucose testing systems reporting that 78% of the hospitals in their U.S. customer base have adopted TGC protocols, and estimating that similar trends are prevailing in Europe.
A new concept for whole blood glucose self-testing was introduced at the MEDICA exhibition by GlucoTel Scientific (Reno, Nevada/Lichtenfels, Germany). GlucoTel is the medical division of the IT security company Safe-com GmbH & Co. (also Lichtefels), and in addition to glucose monitoring technology is also focused on telemedical applications for monitoring of blood pressure, body weight, and other parameters.
The company has developed a new glucose meter that communicates via Bluetooth to any enabled cell phone, as well as Java-based software that runs on the phone and provides a wireless link to the Internet and thence to a disease management website running on a dedicated server.
The system provides automatic archiving of glucose readings as well as of patient-entered data on exercise level, food intake, drug dosage, and other factors relevant to diabetes management such as intake of nutritional supplements. The web site provides a graphic representation of the archived data, and allows review via the Internet by the patient and his or her doctor or diabetes educator.
GlucoTel has already received FDA clearance and CE-marking for the test strips used with the system, which will represent the primary revenue source for the product line, and it is applying for clearances for the meter and software, with a target worldwide launch in Q107. The company may enter into a partnership with a telecommunications provider to obtain a source for the cell phone used with its system.
GlucoTel also is drawing upon the resources of its parent to provide highly secure, encrypted, HIPAA-compliant data communications and archiving, which is a particularly important requirement in the European market. The company plans to price its meter competitively, and believes the test strips will be priced at below the market average.
Glucose meters — and more
Similar products that combine a glucose meter with a cellular phone to transmit glucose readings to a server are manufactured by Infopia (Anyang, Korea), Hahn & Hahn (Waiblingen, Germany), eHIT (Kuopio, Finland). Cardiocom (Chanhassen, Minnesota), through its newly formed GlucoCom division, has also introduced a telemedicine product for glucose monitoring as a new component of its Autlolink Diabetes Telemonitoring System.
Hahn & Hahn's MWD Diabetes Management System consists of a highly compact meter, the MWD Pen Sensor, and a handheld wireless communication device, the Medwatchdog. Up to 10 glucose readings can be stored in the Pen Sensor. Stored glucose test data is uploaded by placing the Pen Sensor in contact with the Medwatchdog. The user can then transmit the archived glucose readings along with data entered manually into the Medwatchdog on physical activity, food and drug consumption, and parameters such as blood pressure readings to a web site for review by a physician.
eHIT is developing the Health Gateway, consisting of a handheld mobile telemedicine unit that can acquire data from a variety of testing devices including glucose meters, weight scales, pulmonary function testing devices, respiration rate monitors, and ECG monitors, and transmit the data to a server via the cellular network using GPRS/3G technology. Data is transmitted in a secure format and can be transferred to an electronic patient record or hospital information system, allowing physician review. Wireless data links to measurement devices are established automatically using the eHIT intelligent Bluetooth adapter, which mates to the measurement device.
In addition to monitoring of blood glucose data, the system has also been configured to monitor coagulation (PT/INR) self-testing data measured using the CoaguChek meter and test strips from Roche Diagnostics (Basel, Switzerland). The software for the system is priced at €1140.
National Diagnostic Products (Sydney, Australia) introduced a new 60-second personal diabetes screening test that does not require a meter for read-out and costs 30% less than a conventional diabetes test strip. The Betachek Diabetes Test, designed for home use, was launched in Australia earlier this year. The product complements National Diagnostic's Betachek Visual Kit, which provides a semi-quantitative visual readout of blood glucose levels using a strip format similar to that used in urine test strips. The latter product is sold in 50 countries worldwide.
The new personal diabetes test is designed for use at home by individuals who have not been diagnosed with diabetes but who are at risk based on age, body mass index, family history, or other factors, and exhibit symptoms of the disease. It is intended to address the issue of undiagnosed diabetes, a significant public health issue because up to 50% of people who have diabetes are unaware of their disease.
HbA1c testing
Another segment of the POC testing market focused on diabetes management is point-of-care glycated hemoglobin/HbA1c testing products. A number of products are already on the market, including the DCA 2000 from Siemens/Bayer Diagnostics (Tarrytown, New York); the Micromat II from Bio-Rad Laboratories (Hercules, California); the A1CNow+ single-use OTC device from Metrika, now a unit of Bayer Diagnostics; the GDX System from Cholestech (Hayward, California); the Nycocard and CLIA-waived Afinion HbA1c tests from Axis-Shield PoC AS (Oslo, Norway); and the €6 Smart/700 HbA1c test from Diazyme Laboratories (San Diego, California).
Another POC system providing HbA1c testing capability, the in2it from Provalis (Flintshire, UK), is no longer actively marketed following the acquisition of Provalis' diagnostics business, PB Diagnostics, by Bio-Rad. A new entrant in the POC HbA1c market, Quotient Diagnostics (Surrey, UK), introduced the Quo-Test A1C hemoglobin A1c testing system at the MEDICA exhibition.
The Quo-Test technology was developed at St. Bartholomew's Hospital (London) and licensed by Quotient Diagnostics. The Quo-Test analyzer employs fluorescence quenching in concert with boronic acid affinity chromatography, creating a homogenous assay technology well-suited to POC testing. Test time is under three minutes not including sample preparation. The test requires a whole blood sample of less than 5 uL.
Two versions of the instrument are in development, including Quo-Test A1C LAB, a compact, dedicated HbA1c reader suitable for low-volume testing in small labs, and the Quo-Test instrument, a $3,000 unit that combines a photometer and a fluorimeter in one unit and is designed for either POC testing or central lab testing.
The latter instrument is intended to serve as a platform that will eventually allow a range of assays to be performed using individual test cartridges. The A1C cartridge will be the first test to be introduced, and feasibility studies have been performed for a total cholesterol assay.
Quotient is targeting a selling price for the HbA1c cartridge of $4 to $5 in the U.S., making the product competitive with existing tests such as the DCA 2000 HbA1c assay that typically is priced at $8-$9. The reader will be priced at $3,000. Quotient is now initiating clinical studies to generate data for a 510(k) submission in the U.S. and CE mark submission in Europe.
The company is targeting product launch during the first half of 200 and is projecting that the system will have CLIA-waived status by July 2007. Quotient believes there is a significant opportunity for expansion of the POC HbA1c testing market.
At present, only around 5 million of the 100 million HbA1c tests performed annually in the U.S. are done POC. In Europe, the company believes that at least one-third of HbA1c testing could migrate to the point of care. Quotient has been funded to date by a combination of venture capital, private investment, and an industry partner, BBI Holdings.
Other companies developing POC HbA1c testing products for the global market include Audit Diagnostics (Cork, Ireland), Nano-Ditech (Monmouth Junction, New Jersey), and Sand County Biotechnology (Taiwan).
Audit Diagnostics exhibited the Liqui-Stat, a development-stage POC wet chemistry analyzer targeted for launch in April 2007. The Liqui-Stat requires a serum or plasma sample, and requires a starting blood sample volume of 10-20 uL. Test time is very rapid, at about one minute, although the initial centrifugation step required for all assays takes about five minutes. Audit plans to offer a broad test menu of clinical chemistry tests, in-cluding HbA1c, as well as immunoassays, drugs of abuse tests, cancer markers, cardiac markers, and electrolytes.
Nano-Ditech has developed a new im-munoassay technology employing microfluidics and electro-immunochromatography (Electro-Immuno chromatography Lab On a Film Chip or EI LOFC). A U.S. patent application has been filed on the technology, which employs a microchannel and conductive particles to provide rapid, quantitative detection using a low-cost chip.
The company has already introduced qualitative immunochromatography test cartridges for POC drugs of abuse and cardiac marker testing. The new HbA1c test cartridge will employ the LOFC technology, providing a rapid quantitative readout. A target launch date has not yet been set.
Nano-Ditech also is developing a troponin I cardiac marker assay employing LOFC technology. Sand County Biotechnology plans to introduce an 8-minute, non-instrumented semi-quantitative test for glycated hemoglobin in whole blood in June 2007. The test cartridge will require application of two drops of blood, one for measurement of total hemoglobin, the second for measurement of non-glycated hemoglobin, with the glycated hemoglobin result derived from the difference between the two measurements.
Highlighting cardiac markers
Another segment of the POC testing market highlighted at the MEDICA exhibition was cardiac markers. The POC cardiac marker segment has attracted considerable investment due to its high growth rate and is expected to total more than $300 million worldwide in 2006, including markers such as BNP and NT-proBNP as well as troponin, CK-MB and myoglobin.
Biosite dominates the market with an estimated 60% share worldwide in 2005, followed by Roche Diagnostics with reported 2005 sales of its Cardiac Reader reaching CHF61 million (about $49 million). At MEDICA, Roche exhibited a new version of the Cardiac Reader, the Cobas h232, that will be launched in Spring 2007. The new reader is designed as a handheld unit, and is slightly larger than the i-STAT analyzer marketed by Abbott Diagnostics (Abbott Park, Illinois), another POC testing system that offers cardiac markers.
The reader features a touchscreen interface, as well as enhanced connectivity. The menu will include NT-proBNP, D-dimer, myoglobin, Troponin T, Troponin T Sensitive, and CK-MB. Roche will target users in the hospital bedside, emergency department, and physician's office segments.
AMIC (Uppsala, Sweden) exhibited the Forecast System, a development-stage POC analyzer slated for initial market launch in late 2007 in Europe. The system is based on AMIC's 4castchip technology, which employs a highly ordered array of micropillars that drive capillary flow of sample and reagents. Key features of the technology include high sensitivity and precision.
The cardiac Troponin I assay under development for the Forecast system has an analytical sensitivity of < 0.05 ng/mL, qualifying it as a high-sensitivity troponin assay. Total test time is 10 minutes, which will allow users to meet the newest guidelines of the European Society of Cardiology (Sophia Antipolis, France) and the American College of Cardiology (Washington) for management of acute myocardial infarction.
Nanomedics Technology (Düsseldorf, Germany), another development-stage company targeting the POC cardiac marker market and founded in 2002, is a subsidiary of Diagenics (Woburn, Massachusetts). Diagenics has introduced the Diacordon laboratory-based ELISA test for the cardiac marker Glycogen Phosphorylase isoenzyme BB (GPBB) in Europe, and its Nanomedics division is now developing a POC version of the assay, as well as a troponin I assay and other cardiac marker tests, based on a biochip platform.
Preliminary studies indicate that GPBB is elevated earlier in myocardial infarction than the troponins and is more specific for MI than myoglobin.
Another POC cardiac marker system was exhibited at MEDICA by Mitsubishi Kagaku Iatron (Tokyo). The Pathfast system is a compact desktop automated immunoassay analyzer which combines chemiluminesence with Mitsubishi's Magtration magnetic particle separation technology. Both whole blood and plasma samples can be used.
Turnaround time for analysis of six samples is less than 17 minutes, depending on the tests performed. The test menu includes Troponin I, myoglobin, CK-MB, D-Dimer, and a new NT-proBNP assay. A total of 80 analyzers have been placed in Japan, and 60 have been placed in Europe to date. Sales in the U.S. are targeted for this month, pending FDA clearance. The Pathfast is sold through a worldwide network of 20 distributors.
POC coagulation
Another growing segment of the POC testing market is products for point-of-care coagulation testing. This market is particularly strong in Europe, where a patient-driven movement originating in Germany has created a high level of awareness of the benefits of regular PT/INR monitoring for patients undergoing anti-coagulant therapy, and many patients now perform coagulation self-testing.
A recent meta-analysis published in the Lancet by Heneghan et al. demonstrates that patient self-testing can reduce thromboembolic events in patients undergoing anticoagulation therapy by 55%, and can reduce mortality by one-third.
The global market leader, Roche Diagnostics, sells the CoaguChek S for professional use in Europe and has recently launched the CoaguChek XS, a compact handheld meter with a 60-second test time, a 10uL blood sample volume requirement, and off-meter dosing. The Roche CoaguChek is also used for patient self-testing in Europe.
Inverness Medical (Bedford, UK), one of the leading suppliers of POC testing products worldwide, introduced a new entry in the market, the SmartCheck INR, designed for patient self-testing. The SmartCheck requires only a 3 uL blood sample, and fits easily in the hand. It is being launched first in Germany, and is priced at €800. Test strips cost €3 each.
Personalized patient monitoring
The market for patient vital signs monitoring technology is also expanding in areas outside of the traditional hospital setting in Europe and worldwide. Wireless technology is playing a major role by allowing patients to be monitored at any location in the hospital as well as after discharge in the home. An important area of focus for new product development in hospital-based patient monitoring is systems to improve the integration of the various aspects of patient management, including both monitoring and therapy, as the patient moves through the care process within the hospital.
At present, most hospitals have a complex and diverse array of instruments in the various specialty departments with differing user interfaces and operating standards.
Many medical errors are attributable to communication problems between departments or different medical teams that manage the patient at various times during a hospital stay. Hand-off of patients between departments or different patient management teams is particularly error-prone.
A new system designed to address such issues was introduced by Dräger Medical (Lübeck, Germany) at the MEDICA exhibition. Key components of Dräger's Infinity Acute Care System include the Medical Cockpit, a standardized control unit, and a personal vital signs monitor that travels with the patient throughout the hospital stay. The monitor employs wireless local area network technology to communicate with the hospital's clinical information system as well as with various fixed base monitors and therapeutic devices such as ventilators, infusion pumps, and anesthesia machines.
In a typical scenario, the TeleSmart personal monitor would be attached to the patient upon initial presentation in the Emergency Department. When the patient is subsequently admitted for treatment, such as for emergency surgery in the OR, the monitor travels with the patient and can be interfaced to equipment such as an anesthesia machine.
To further simplify the transfer process, Dräger has developed a single-use ventilator tubing set that contains an integrated electronic chip which can automatically transfer ventilator settings used in the ED to the ventilation/anesthesia equipment in the OR, eliminating the need for time-consuming and error-prone data re-entry.
When the patient is subsequently transferred to the recovery room, the personal monitor again travels with the patient to ensure continuity of tracking of physiological status, and to automatically transfer patient information to fixed-base equipment such as ventilators or drug delivery devices. If the patient is then transferred to the intensive care unit, the personal monitor continues to stay with the patient for transfer of data and continuous monitoring without the need for manual information exchange or data entry. The Infinity System not only tracks clinical parameters, but can also be used to monitor the cost of care throughout the hospital stay.
Dräger also exhibited a product under development for non-invasive monitoring of lung respiratory volume that allows ventilator parameters to be determined using electrical impedance tomography. Feasibility studies with the device are nearly complete, and according to the company physician feedback has been positive. The respiratory volume monitor is about two to three years away from market introduction.
Out-of-hospital monitoring
A number of companies exhibited new devices for monitoring of patients outside of the hospital setting, in keeping with the growing trend to deliver care in the least expensive setting while maintaining the capability to continuously track key patient data.
Biomedical Instruments (Shenzhen, China) exhibited the MOQI ambulatory wireless monitor, which allows tracking of ECG, non-invasive blood pressure, oxygen saturation, respiration rate and temperature remotely via a link to the cellular telephone network.
The monitor can also be configured to communicate via wireless LAN, or a memory card can be inserted to record patient data. At present, the device, which is priced at $3,500, is sold only in China for use in emergency departments and research hospitals, but the company is seeking distributor partnerships to market the product worldwide.
Cardiomedix (Evanston, Illinois) has introduced the Physio-Glove-ES/ET in Europe, used for remote monitoring of 12-lead ECG data. The system consists of a glove containing a network of ECG leads that is placed over the patient's chest, and a Bluetooth interface for transmission of ECG data to a cell phone, from which it can be uploaded to a monitoring center. The company has submitted to the FDA for 510(k) clearance. Cardiomedix provides a monitoring service and also sells a $2,000 software package allowing a monitoring center to be set up in a nursing center or clinic.
In addition, the company markets the Health-e-Chair, a specialized chair outfitted with monitors for body weight and blood pressure, with an interface that connects to the patient's television to interact with a care manager in the monitoring center. The complete monitoring service using both the Health-e-Chair and the Physio-Glove has a base price of $250 per month, adjusted based on usage level.
Energy Lab Technologies (Hamburg, Germany) introduced the viport ECG event recorder at the MEDICA exhibition. The device is a further development of the viport mini-ECG device launched last year, which is a self-contained handheld unit that performs three-lead ECG measurements. The new model with event recording also includes a real-time color display of the ECG trace, and can display a color-coded electrocardioportrait indicating heart function and cardiac stress.
Cardiac stress is derived from measurement of heart rate variability, and can indicate the presence of diseases other than arrhythmias such as infections and mental disorders. Two versions are available, a €399 consumer model for home use by patients, and a €599 professional model with added software capabilities. Physicians can use the viport in the home or office to acquire ECG data on the spot in about two minutes. The data can be transferred via Bluetooth link to a PC or cellular phone for transmission to a monitoring center.
Home and alternate-site treatment advances
Other new developments were exhibited at MEDICA in the areas of respiratory disease management and diabetes management.
A new instrument for rapid POC screening for tuberculosis infection was previewed at the MEDICA exhibition by Clement Clarke International (Essex, UK). The XTS Xpress TB Screen is a breath analyzer that provides detection of patients infected with tuberculosis in under five minutes.
The system consists of a disposable breath collection device that is coated with an antibody that specifically binds to tuberculosis mycobacteria, which are expelled in the breath of individuals with active, contagious infections. After collection of a breath sample, the collection device is inserted into a handheld analyzer that detects presence of tuberculosis bacteria via a fluorescent labeling technique.
The product has already received 510(k) clearance in the U.S., and CE marking is pending. The target date for market introduction is April 2007. Cost for the analyzer is about $8,000, with the consumable collection device priced at $10. In an initial trial conducted in India, the device exhibited 100% sensitivity for detection of tuberculosis infection. Potential applications include screening in immigration control, primary care diagnosis, containment of outbreaks in high risk areas, and screening of patients in clinical trials.
SeQual (San Diego, California) exhibited the Eclipse oxygen concentrator, a portable device that is about the size of a small carry-on suitcase and can operate on a rechargeable internal battery to provide oxygen therapy during short periods of transport. It is approved for use aboard commercial airplanes.
The Eclipse was launched in the European market just prior to MEDICA, and was introduced in the U.S. in August 2006, where it has already been deployed by more than 200 providers serving in excess of 60% of the 1.5 million existing home oxygen patients.
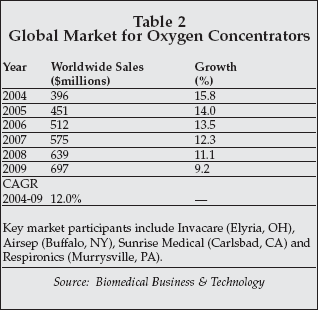
The product addresses the growing market for oxygen concentrators, which, as shown in Table 2, exceeded $450 million worldwide in 2005. In the U.S., changes in Medicare reimbursement for oxygen therapy allowing payment for therapy delivered by portable devices will drive expansion of the market for products such as the Eclipse. The Eclipse can deliver continuous oxygen at a 3-liter-per-minute rate, and can eliminate the need for oxygen cylinders for most patients.
 |
Delivering insulin
A new development in insulin therapy for diabetes was announced by Wristop Technologies (Vantaa, Finland), which manufactures wireless wrist computers and wearable and portable medical and leisure instruments. At the MEDICA exhibition, the company introduced the WRISTOP wireless insulin pump controller. The battery-powered device is the size of a typical wrist watch, and can be customized with different colors and designs for various user segments such as children. It internally measures energy consumption of the wearer as well as heart rate, and can interface to a glucose meter via Bluetooth or an infrared link.
In addition, the device can be connected to continuous glucose sensors manufactured by Roche and Medtronic (Minneapolis). Via a wireless interface to the user's insulin pump, the device can display the level of insulin remaining in the pump, and allows the user to remotely set the insulin dose and activate the pump from the WRISTOP, avoiding the need to access the pump directly. That factor is important, according to Wristop, because it allows the user to be less conspicuous when operating the pump. Cost has not yet been set. The company plans to establish partnerships with insulin pump manufacturers to commercialize the device.
gbo Medizintechnik (Rimbach, Germany) exhibited the HiToP electrotherapy system for treatment of diabetic neuropathy. Existing therapies for diabetic neuropathy such as pain medications have limited effectiveness, as does transcutaneous electrical nerve stimulation (TENS) therapy.
The HiToP system, which is available as both a professional-use model for physician's offices as well as a home-use model, employs higher frequency stimulation versus TENS.
According to the company, high frequencies allow energy to be coupled into the body more effectively. A comparative study using the HiToP system versus TENS demonstrated an 80% response rate in diabetics treated with HiToP compared to 30% for TENS. Therapy is applied daily and typically must be continued indefinitely.
However, there is some evidence for improvement in microvascular perfusion in treated patients, presumably reflecting restoration of microvascular endothelial cell function, as well as evidence for reduction in insulin requirements for diabetics who take insulin. The HiToP system is sold in Western Europe and some parts of East Europe, as well as in Japan and Korea. gbo Medizintechnik is now in the process of applying for FDA clearance to allow marketing of the product in the U.S.
