Twenty-eight years after the scanning tunneling microscope (STM) was invented at IBM (Armonk, New York) enabling the advent of nanotechnology research and earning its inventors the Nobel Prize, a new group of scientists there has adapted an iteration of that technology so powerful that they are able to see the anatomy of a single molecule for the very first time.
This achievement, reported on Friday in Science, was actually a stunning surprise to the scientists involved.
"This happened by chance," Fabian Mohn, a scientist at IBM Research (Zurich, Switzerland), told Medical Device Daily. "We were working toward this and it was known from previous work that the tip modifications to the atomic force microscope can be done, but we didn't know it would increase the resolution so much."
The atomic force microscope (AFM) is an offspring of the original STM. Mohn and his group were able increase the sensitivity of the microscope and establish the required ultra-cold atmosphere of minus 451 degrees F so that human eyes could finally see atomic structures that are the backbones of individual molecules.
Mohn, who is working on his PhD thesis, was part of a team at IBM led by Leo Gross. In June, the same group of researchers measured the charge states of atoms using an AFM. Both breakthroughs are important to the eventual development of smaller, faster and more energy-efficient computing components, including medical devices.
Here's how they did it:
For this experiment, the team chose to look at a pentacene, an oblong organic molecule that consists of 22 carbon atoms and 14 hydrogen atoms measuring 1.4 nm. In the image provided by Mohn (See Figure 1), the hexagonal shapes of the five carbon rings as well as the carbon atoms in the molecule are clearly resolved.
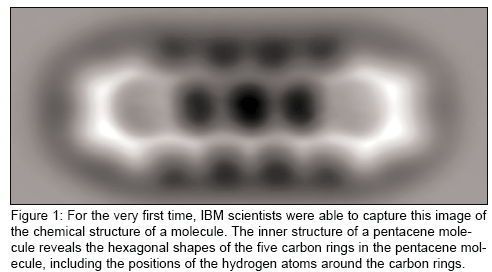
"In AFM, you have a very sharp tip that is scanned above the sample," Mohn said. "The signal that's used to image the sample, in this case the molecule, is generated by the force between the tip and the sample.
"The crucial part in this work to achieve high resolution is that we were able to build up the tip in a controlled way," he said. "We used previously established atom manipulation techniques to pick up single atoms or molecules arranged at the tip."
But to do this, they had to work in extremely close proximity to the molecule – less than one nanometer. But getting that close usually causes the molecules to be displaced in the same way that magnets repel each other.
A key part of the experiment was keeping everything mechanically stable.
"Each item has to stay exactly where it is otherwise the measurement will be spoiled," Mohn said. "So we work at the temperature of liquid helium of minus 450 degrees F."
The scientists then made a 3-D map of the molecule investigated. It took 20 hours of data acquisition, with the microscope stable and the temperature beyond chilly, to grab the image.
"You can see the image and rings of carbon atoms. Of course, it wasn't clear before that it would look exactly like this," Mohn said. "But then it was clear it was a pentacene molecule."
"The microscope isn't optical. In fact the human eye can't look through it to actually see the molecule. It takes an electrical signal generated by force and it's displayed on a computer image. This is not possible to directly view such small structures because wavelengths of light are too long."
The group has been able to repeat the procedure dozens of times. "We don't have a 100% rate of success, but we can repeat this," he said.
In the future, these single molecules will play a central role in electronics.
"In order to get there it will be crucial to know the exact geometry, to know how molecules absorb to an electrical contact," Mohn said. "This imaging technique opens up possibilities of investigating further at the atomic level. It's very fundamental research."
Next, the team plans to investigate how this imaging contrast is generated.
"To be more specific, we want to see the role of chemical composition of the molecule," he said. "We had molecules of carbon and hydrogen. What happens if we replace carbon with oxygen? Would it look different? We'll examine chemical sensitivity. We are also interested in the charge distribution in single molecules. Imaging the distribution of electron charge would be of great interest, especially in terms of future molecular electronic applications."
IBM has invested a great deal into the exploration of molecules, atoms and the study of nanotechnology. Eventually this work is intended to interconnect instruments and the physical world. The company is currently building the Nanoscale Exploratory Technology Laboratory on the campus of IBM Research in Zurich with plans to open in 2011.
Lynn Yoffee, 770-361-4789; lynn.yoffee@ahcmedia.com
