BB&T Contributing Editor
LOS ANGELES — Interventional oncology, the treatment of cancer with a device-based approach, is a fledgling subspecialty within the field of minimally invasive medical devices. These procedures are typically performed by interventional radiologists, who utilize image guidance and minimally invasive devices and procedures to treat cancer in the least invasive way possible.
Interest in this new field is clearly increasing. The first meeting of the World Congress of Interventional Oncology (WCIO; Beverly, Massachusetts) took place in 2005 in London, and attendance has increased steadily with improving technologies and the desire by patients and insurers to avail themselves of less-invasive approaches. Attendance was at a record level at this year's meeting, held for the second time in the U.S.
As the industry leader in the broader field of interventional radiology (IR), with annual sales in its fiscal year ended May 31 of about $167 million, it was no surprise to see the significant presence of AngioDynamics (Queensbury, New York) at the meeting. With the strategic acquisition of RITA Medical (Fremont, California) for about $225 million in January 2007, AngioDynamics instantly became a major player in interventional oncology.
Its major products in this area, all acquired via the RITA deal, include radio frequency ablation (RFA) devices, the Habib line of conventional and laparoscopic surgical resection devices and embolic microspheres, which are also called drug-eluting beads (DEBs).
These latter two product lines were distributed by RITA under exclusive licensing deals prior to the AngioDynamics purchase. The Habib bipolar resection device, which uses RF energy has been widely used in liver cancer resection procedures and has proven to decrease operative and anesthetic time as well as blood loss.
Drug-eluting beads have the ability to actively sequester a chemotherapeutic agent (for example doxorubicin) from solution and release it in a controlled and sustained fashion into a cancerous tumor. Clinicians often refer to this modality as transcatheter arterial chemo-embolization (TACE).
Whereas Habib and DEBs are relatively new to the field of interventional oncology, for more than a decade, RFA has been used to treat tumors that were considered unsuitable for conventional forms of treatment such as surgery, radiation, or chemotherapy.
A well-attended AngioDynamics evening symposium, titled "Novel Approaches in the Management of Primary and Metastatic Cancers: Thermal and non-Thermal Ablative Techniques: DEBs and TACE," addressed the use of RFA and DEBs in oncology, as well as the company's newest and potentially most significant technology, irreversible electroporation (IRE).
Riccardo Lencioni, MD, an interventional radiologist from Cisanello University Hospital (Pisa, Italy), cited two key studies that validate the thesis that an interventional approach to cancer therapy is effective. Leoncini, one of the world's authorities on RFA, described the AngioDynamics' DEBs, trade-named LC Beads, for intra-arterial injection. He reported on a pilot study, conducted in Italy, of 20 patients with liver cancer which showed that intra-arterial administration of DEBs clearly enhanced the effect of RFA. This regimen led to a high rate of sustained complete response in tumors that had been resistant to standard RFA treatment.
He noted that this small trial showed the first evidence of the synergy between RFA and a controlled, sustained local delivery of a chemotherapeutic agent in human cancer treatment. More importantly, he said that that the combination of DEBs and RFA "has the potential to become the standard approach" for treating certain liver cancers. The full results of this study appear in an article titled "Doxorubicin-eluting bead-enhanced radiofrequency ablation of hepatocellular carcinoma: A pilot clinical study" in the August issue of the European periodical Journal of Hepatology.
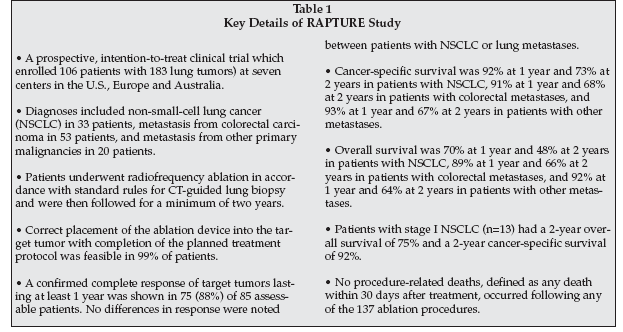
Leoncini also provided information on the landmark Radiofrequency Ablation of Pulmonary Tumors Response Evaluation (RAPTURE) trial, which is believed to be the largest study ever to evaluate RFA for treating lung cancer. According to the American Cancer Society (Atlanta), about 161,000 Americans will die from lung cancer in 2008. It is the leading cause of cancer death in U.S.
Some of the details of the study, which were published in the July 2008 issue of The Lancet Oncology are provided in Table 1. In commenting on the trial, Leoncini said that "RFA could become a treatment option for all patients who cannot have standard surgery, whether they have concomitant chemotherapy and radiotherapy or not."
Robert Martin, MD, a surgical oncologist at the University of Louisville, further supported the use of LC Beads for liver cancer in an informative talk. He noted that while there are a plethora of options to treat liver cancer (see Table 2), the use of drug-eluting beads affords the advantages of more precise drug delivery and consistent results with significantly reduced patient side effects.

Martin reported the first results of an LC Bead registry, which was launched over a year ago and to date has enrolled over 100 patients at 11 active U.S. sites. It is currently enrolling liver cancer patients, the vast majority of whom have already failed first-line chemotherapy, with the goal of embolizing the vessels which are feeding their hypervascularized tumors. Physicians are employing two different chemotherapeutic agents — doxorubicin for liver and most other cancers, and irinotecan primarily for metastatic colorectal cancer.
Martin indicated that the result of the registry so far is showing that most patients are experiencing a "dramatic response rate." He said that "this is a viable drug-delivery concept, is safe and effective and may represent a paradigm shift." However, he also sounded a cautionary note, saying that "we still have many more questions than answers about how this will fit into the management of our cancer patients."
Surgery at the cellular level
The final two talks at the AngioDynamics symposium were devoted to a detailed discussion of its exciting new technology, irreversible electroporation. In introducing this new concept to the interventional oncology community, AngioDynamics describes it as "NanoKnife Surgery at the Cellular Level."
IRE energy is delivered through needles and image guidance very similar to existing ablative technologies such as radio frequency or cryothermia but instead of heating or freezing to kill the targeted tissue, it uses electrical fields to cause cell death. The powerful electrical field, which passes through an electrode array, causes permanent nanoscale defects or pores in the cell membranes. The permanently impaired cells, killed through the process of virtually instantaneous apotosis are then removed through the body's natural immune system.
As compared to other ablation methods, it is highly selective and does not damage adjacent critical structures such as ducts and blood vessels. It appears at this early juncture that it could have very broad application within the field of tissue ablation, spanning benign tissue removal such as BPH and malignant tissue represented by cancer.
The technology was developed at the University of California, Berkeley and then licensed to a private company called Oncobionic. In October 2006, AngioDynamics signed an agreement to acquire Oncobionic, making an initial investment of $5 million and a commitment to pay an additional $20 million within two years, subject to successful human use of IRE.
In April, AngioDynamics reported that the first human clinical cases, treating prostate cancer, had been performed and that it was moving forward with its plans to complete the acquisition of Oncobionic. As per the agreement, an additional $10 million was paid, with two more payments of $5 million each scheduled within the next 18 months.
Two speakers fluent about IRE discussed its attributes at the evening symposium. Stephen Kee, MD, associate professor of radiology at University of California Los Angeles (UCLA) noted in his talk that IRE has been thoroughly investigated at his institution from a variety of standpoints. A series of key questions (see Table 3) have been investigated and appropriately answered and now the company will be aggressively moving forward with both animal and human trials.

The university has funded a two-year animal trial, which began in September 2007. A tumor is implanted in rabbits, with one-third followed up as controls, one-third treated with RFA and one-third treated with IRE. The endpoint will be the growth rate of the tumors, or lack thereof.
According to Kee, IRE has several important advantages, including its very short procedure time, its larger ablation zone, the absence of a heat-sink effect and its ability to be monitored with ultrasound in real time.
IRE has broad application within the field of cancer, but given the large number of prostate cancer cases that occurring annually, the company has opted to begin working with this area. Six human prostate cancer cases have been performed to date, taking advantage of a 510(k) approval received in November 2006 for soft tissue ablation. AngioDynamics is planning to proceed with a prostate-specific clinical trial, first treating animals and then moving into human trials.
In its recent 4Q08 conference call with analysts, the company indicated that it hopes to begin placing the first 20 NanoKnife units with "thought leaders" in the U.S. and Europe. It also noted that a clinical trial will begin soon in Italy and that additional prostate cancer patients will be treated in the U.S. AngioDynamics said it expects to invest $5 million in its current fiscal year ending May 31, 2009, in developing and commercializing IRE.
The final speaker was Gary Onik, MD, who is one of the three founders of Oncobionic and also has been a driving force in minimally invasive cryoablation of the prostate.
Onik, an interventional radiologist from Celebration Health Florida Hospital (Celebration, Florida), is widely recognized as one the earliest adopters in the field of interventional radiology. Onik compared surgery for women with breast cancer and surgery for prostate cancer. He contended that breast surgeons have shown that a minimally invasive lumpectomy, which spares breast tissue, can be as effective as a more aggressive procedure.
Prostate cancer patients can be safely treated with a "middle ground" between the two extreme methods of treating prostate cancer the gold standard of radical prostatectomy and the more controversial and passive approach of "watchful waiting." Armed with data from several studies, Onik suggested that about two-thirds of all prostate cancer is amenable to a less-invasive approach, which he calls the "male lumpectomy."
He disclosed the results of his series of 120 focal cryoablation patients, which demonstrated excellent efficacy and reduced morbidity, especially as it relates to urinary incontinence and sexual potency.
As portrayed in Table 4, on the next page, Onik noted that IRE has numerous potential advantages in treating prostate cancer and relatively few drawbacks. The early human data in the treatment of prostate cancer has been very encouraging, with negative biopsies and full continence and potency for all six patients. He did note that there are potential disadvantages that remain, including "the great unknowns," i.e., finding a problem or challenge that heretofore has not been addressed or surfaced.

Onik concluded by saying, "While we have a lot more work to do on IRE to establish its specific role, I am very excited about its potential."
An intriguing variation on the theme of attacking cancer cells through interventional means was presented at a main WCIO session by Tony Reid, MD, associate professor at the University of California, San Diego. He discussed the use of a breakthrough product class of cancer therapeutics vaccinia viruses that have been engineered to target, attack and eradicate cancer without harming the surrounding cells.
These agents are derived from a proprietary class of targeted and armed oncolytic poxviruses. They can multiply selectively within cancer cells, leading to their destruction. These newly created copies are then released and are able to infect and eradicate other tumor cells both locally and in distant sites in the body. This cycle of replication, cancer cell destruction, release and spread is then repeated. Normal cells are not affected, resulting in safety and tolerability.
The developer of one of these products, Jennerex Biotherapeutics (San Francisco), has been reporting data on its virus JX-594 at recent medical conferences. Preliminary Phase I data, which were published in the June issue of The Lancet Oncology, in an article titled "Use of a targeted oncolytic poxvirus, JX-594 in patients with refractory primary or metastatic liver cancer: a Phase I trial," showed strong evidence of safety and efficacy. The 14 patients who were treated in this trial received an ultrasound-guided intratumoral injection of JX-594.
Reid said that JX-594 early results are "extraordinarily good" and that this approach could potentially become a "major weapon" against cancer, especially lung and liver. A larger Phase II trial is now under way.
Emphasis on image guidance
One of the recurrent themes of this conference was the need for better image guidance, which cast the privately-owned company Traxtall (Toronto) in the limelight. Its FDA-approved PercuNav system guides a needle or probe to any pre-defined target using pre-operative or intra-operative images derived from CT, MRI or ultrasound.
By tracking the tip of either rigid or flexible instruments using tiny sensors embedded in the instruments, its system provides real-time 3-D visualization and navigation during RFA and other interventions such as biopsies, ultrasound trackers and probes. The system transforms 2-D patient images into dynamic maps showing the location of the instrument in the patient.
The company refers to its system, which sells for about $100,000, as a "GPS system for the human body." It has recently placed systems at several prestigious sites such as Massachusetts General Hospital (Boston) and the National Institutes of Health (Bethesda. Maryland) and has several more key hospitals in its pipeline that will soon be accepting shipment and installation. The company is now ramping up its sales force for a big commercial selling effort.
