Biomedical Business & Technology Contributing Editor
DUSSELDORF, Germany — The MEDICA exhibition, held here in mid-November, is the largest forum in the world for introducing new medical device technologies and thus provides a window not only on new trends in product development but also on global competition in the medical device industry. New developments in patient monitoring technology, including technology for remote monitoring of patients outside the hospital, and expansion of monitoring within the hospital, were introduced by numerous companies.
In addition to the many companies at the exhibition, based in Europe and the U.S., an increasing number of Asian companies come to this high-tech gathering, demonstrating the global level of competition in the medical device market.
Another segment experiencing increasing competition, as well as a growing level of investment, is point-of-care (POC) testing, a segment that is being driven by demands for improved efficiency in patient management and by calls from patients themselves for better access to healthcare, along with a greater level of involvement in their own care.
Other areas highlighted at the MEDICA exhibition included diagnostic imaging and minimally invasive surgery, including new devices employing advanced biomaterials for improved performance. Still another topic increasing in importance in both Europe and the U.S. is prevention of infection in the hospital setting.
New Medicare reimbursement initiatives in the U.S. scheduled to take effect in 2008, under which Medicare will no longer reimburse hospitals for the cost of treating infections acquired after admission — coupled with an increasing awareness of the costs and adverse events associated with infection in the hospital — are leading to demands for improved approaches to infection prevention and more effective technologies for wound management.
Non-invasive monitoring gains
A number of companies introduced new non-invasive monitoring products at the MEDICA exhibition, demonstrating the continuing trend toward reduced invasiveness in medicine.
Cheetah Medical (Indianapolis/Tel Aviv, Israel) launched a new non-invasive cardiac output monitoring system, the Reliant, which is a second-generation product aimed at clinical (vs. research) applications, with a specific focus on monitoring of intensive care patients.
While a number of non-invasive cardiac output monitors are already on the market, their acceptance in the ICU and CCU setting has historically been limited because of the difficulty in producing accurate and reliable measurements in severely ill patients.
The Reliant is based on Cheetah's BIOREACTANCE technology, which employs a new approach for performing non-invasive cardiac output measurements that combines bioimpedance, a technique employed in other non-invasive monitors such as the BioZ system from CardioDynamics (San Diego), with bioreactance (frequency-based measurements), allowing analysis of both amplitude and phase of bioimpedance signals. The use of phase data significantly improves accuracy, according to the company, and also reduces the dependence of the readings on electrode placement.
The Reliant system uses four electrodes, typically placed on the patient's upper torso, plus a conventional blood pressure cuff to provide continuous non-invasive measurement of cardiac output and blood pressure.
In addition to applications in monitoring of ICU and CCU patients, the Relianct also has applications in monitoring of patients in step-down departments, in peri-operative monitoring, and in the emergency department. Cheetah is an angel-funded company founded in 2002. The Reliant system has CE marking but has not yet received FDA clearance.
CNSystems (Graz, Austria) exhibited the CNAP Monitor 500, a continuous non-invasive arterial pressure monitor that employs a fingertip pressure sensor which measures the arterial pressure wave along with a conventional blood pressure cuff for pressure calibration. The CNAP analyzes signals from the fingertip sensor to provide a real-time display of the arterial pressure waveform.
According to the company, 85% of cardiac surgeries are performed using only conventional non-invasive blood pressure monitoring, and the new CNAP monitor will now enable tracking of the pressure waveform, which previously was only possible if an invasive pressure monitoring catheter is inserted. The technology was introduced at the 2006 MEDICA exhibition as the Infinity CNAP SmartPod module, integrated into the Dr ger Medical AG & Co. (L beck, Germany) Infinity multi-parameter patient monitoring system.
Now, a new stand-alone version of the technology, the €9,000 CNAP Monitor 500, has been developed. The new system is expected to have applications in continuous pressure monitoring in the OR during anesthesia procedures and in other short-term hemodynamic monitoring situations.
In-hospital monitoring
Technology for expanded monitoring of patients in the hospital was introduced commercially for the first time at the MEDICA fair by Emfit (Vaajakoski, Finland). The company's DVM (Discrete Vitals Monitoring) technology measures basic physiological parameters such as heart rate, respiration rate, physical movement/activity passively from below the patient's mattress, without any electrodes, leads, cuffs or cannulas. The system also provides information on patient presence as well as fall alarms (with an optional floor sensor), provides nurse call functions, and in future versions may provide arrhythmia detection.
Emfit exhibited a prototype of the system at last year's MEDICA exhibition, and launched the system worldwide this year. The technology employs a patented thin film dynamic sensor under the mattress, a control unit at each monitored bed, and an interface to a wired or wireless local area network (LAN or WLAN) to transmit the data to a central monitoring station. The DVM system is not intended for use in critical care areas, where patients' vital signs are already routinely monitored, but in the general wards, where continuous monitoring is typically not performed.
Secumatic (the Hague, the Netherlands) introduced a system for patient presence monitoring in the hospital, the SecNurse, which employs cameras placed above each monitored bed along with image recognition software to detect when a patient leaves the bed and sound an alarm either immediately or if the patient does not return within a specified time. The system is simple to install and cost-effective, and, according to Secumatic, provides a higher level of reliability in detecting patient movements compared to bed mats or infrared sensors.
Home monitoring
Remote patient monitoring in areas outside the hospital, including the home, is a growing field in Europe as well as the U.S.
For example, ambulatory blood pressure monitoring is being used for an increasing spectrum of patients, with a goal of improving the control of hypertension and thereby reducing cardiovascular risk. In Germany, only 35% of all patients with hypertension have their blood pressure in control. That percentage can potentially be increased to 80% with effective ambulatory monitoring practices.
I.E.M. (Stolberg, Germany) exhibited the MOBIL GRAPH ambulatory blood pressure monitoring system, which uses a Bluetooth link to transmit ambulatory blood pressure data to the patient's cell phone, and thence to an on-line database on a central server. Software running on the server tracks trends in the data and can return alarms to the patient or send appointment reminders based on the patient's status.
Another version of the system employs a wireless modem in the patient's home that can transmit blood pressure data via the standard phone line. The system has been in use for three years in Germany, and about 10,000 patients are currently using the monitoring service.
I.E.M. is about to initiate a project with the Cleveland Clinic Foundation (Cleveland, Ohio) to telemonitor 5,000 clinic employees and analyze the improvement in blood pressure control achieved with continuous ambulatory remote monitoring.
In Europe, recommendations have recently been published by the European Society of Cardiology (Sofia Antipolis, France) for tele-transmission of home blood pressure data as a means to improve control of hypertension.
POC testing keeps growing
Advances in POC testing were announced by a number of companies at the MEDICA exhibition.
i-SENS (Seoul, Korea) previewed a new point-of-care blood gas/electrolyte/chemistry system, the i-LAB, which will compete with products such as the i-STAT system from Abbott Diagnostics (Abbott Park, Illinois), and is scheduled for launch in 2008. i-SENS currently manufactures a family of whole blood glucose testing systems, including the CareSens Q, offering very competitive features such as a 0.2 uL sample size and two second test time with no coding required.
The company is now expanding its sensor platform to develop the i-LAB, which will first offer electrolyte tests followed by blood gases and metabolites. The compact battery-operated analyzer will have wireless Internet capability for data interface, and will employ multi-test cartridges providing 100 tests each. Test time will be 60 seconds, and required sample volume is 60 uL. i-SENS expects to be able to price the system and associated test cartridges significantly below current levels in the POC testing market because of its low-cost manufacturing capabilities.
Roche Diagnostics (Indianapolis), the leading supplier of POC testing products worldwide, also exhibited a new blood gas/electrolyte test system, the Cobas b123, at the MEDICA fair. The b123 is a multi-test cartridge system that will be targeted at near-patient testing applications in the hospital, and will be launched in late 2008.
The worldwide market for point-of-care testing products used at the hospital bedside, including POC blood gas/electrolyte/chemistry systems, whole blood glucose test systems, POC coagulation testing products, POC drugs of abuse and cardiac marker tests, rapid infectious disease tests, and POC hemoglobin and hematocrit testing products, approached $1.5 billion in 2006, as shown in Table 1, and is forecast to expand at 11.7% annually over the next five years.
 |
POC testing across the spectrum
Another Korea-based competitor in the POC testing market, Infopia (Kyunggi, Korea), unveiled a long-term product development program aimed at building an integrated portfolio of POC testing systems serving the entire spectrum of healthcare delivery sites, encompassing the hospital, clinic, mobile settings such as ships and airplanes, the pharmacy, home nursing, and self-testing.
Infopia completed an Initial Public Offering on the Korea Stock Exchange in June 2007, and currently manufactures a comprehensive line of POC testing products for diabetes management, including the new Evolution blood glucose monitoring system (0.3 uL sample volume, 3-second test time, and autocoding); the Glucose Phone, a combination glucose meter and cellular telephone sold by LG Electronics (Seoul, Korea); and a new combination hemoglobin A1C/glucose POC analyzer offering a five minute turnaround time for HbA1c measurement.
The systems are based on Infopia's nanobio sensor technology. Infopia has supplied glucose testing systems on an OEM basis to companies including B Braun (Melsungen, Germany) and Samsung Electronics (Seoul, Korea).
The company's expanded product line now under development will include POC testing products for management of a wide range of major disease states including cardiovascular disease, cancer, and liver disease.
Infopia will introduce a new cholesterol monitoring system in 2008 along with a diagnostic sensor for liver disease, followed in 2009 by a cardiovascular diagnostic ssensor and in 2010 by a cancer diagnostic sensor.
Enabling telemedicine
In 2011, a remote diagnostic system will be introduced that will employ telemedicine technology to enable on-line interaction of patients with their physician, including analysis of POC test results, counseling, and prescription of medications.
Biochemical Systems International Srl (Arezzo, Italy), another company at MEDICA with a new multi-parameter POC testing system, exhibited the multiCarein, a compact meter that can measure glucose, cholesterol and triglycerides using a fingerstick whole blood sample and individual sensor strips.
A similar product, the Cobas Accutrend Plus handheld analyzer to be launched in January 2008 by Roche Diagnostics, is capable of performing tests for glucose, triglycerides, lactate and cholesterol at the point of care.
In the rapidly growing cardiac marker segment, Diagenics International (Woburn, Mssachusetts) exhibited the DIACORDON POC testing system for early detection of acute coronary syndromes. Diagenics previously introduced a laboratory ELISA test for Glycogen Phosphorylase Isoenzyme BB (GPBB), a marker that provides earlier detection of a myocardial infarction than Troponin I or CK-MB, providing a positive indication of MI within one hour of onset, according to the company.
Diagenics is now developing a multi-analyte biochip sensor that will provide results for eight cardiac markers in a single 15-minute test. Initially, the company plans to launch a GPBB-only sensor, followed by a combination GPBB/Troponin I sensor. Diagenics is also planning to introduce tests for stroke and cancer diagnosis.
Imaging with vibrations and sonics
Various developments in diagnostic imaging were introduced at MEDICA by a number of companies.
GE Healthcare (Milwaukee) introduced a new technology for non-invasive functional imaging of the lungs, Vibration Response Imaging or VRIICU. The system employs sonic analysis, detecting sounds emitted by air vibrating in the lungs and mapping the signals to create a regionalized picture of lung disorders. No energy is applied to the patient, and about 20 seconds is required to obtain an image.
The VRI system can be used, for example, to diagnose collapsed lung, or to assess the degree of lung pathology in patients with respiratory conditions.
A key application is to assess patients on ventilator therapy in the ICU to determine optimal ventilator settings and avoid lung damage.
The VRI system was developed by Deep Breeze, an Israeli company acquired by GE Healthcare. The product is undergoing validation studies in the U.S., with plans for launch in the U.S. market in 2008. Sonosite (Bothell, Washington), the leader in the hand-carried ultrasound imaging market, exhibited its new point-of-care TURBO imaging platform designed to address applications for specific specialties such as anesthesiology (the S-NERVE system), emergency medicine (S-FAST), intensive care medicine (S-ICU), and catheterization laboratories (S-CATH).
The systems are designed for ease of use, and require minimal operator adjustments to obtain a high quality image. They are designed for guidance of procedures such as regional nerve blocks, central line placement, and rapid assessment of internal organs to detect bleeding or diagnose ectopic pregnancy, and are priced beginning at about $50,000.
Another unique POC system was introduced at MEDICA exhibition by InfraScan (Philadelphia, Pennsylvania). The InfraScanner handheld brain hematoma detector is a non-invasive analyzer employing near infrared technology for the detection of bleeding in the brain in head trauma patients. The system analyzes the differential absorption of near-infrared light in bleeding vs non-bleeding tissue in the brain due to hemoglobin. A pilot clinical study conducted on 305 patients at Baylor College of Medicine showed a sensitivity of 100% for detection of extracerebral hematomas and 98% for detection of intracerebral hematomas, with no false positives observed.
The infection fight
New technologies for prevention of infections in the hospital setting were another highlight at MEDICA.Hospital-associated infections (HAIs) are a major issue in healthcare institutions worldwide, as shown in Table 2 (next page) and are the fourth leading cause of death in the WEstern world. HAI is a particularly iportant concerni n Asia, since 90% of hospital-acquired infections in that region are resistant to antibiotic treatment. Given the high cost of treating patients who develop HAIs, hospitals can justify a significant level of investment in infection prevention, and many companies are now introducing products to address the need.
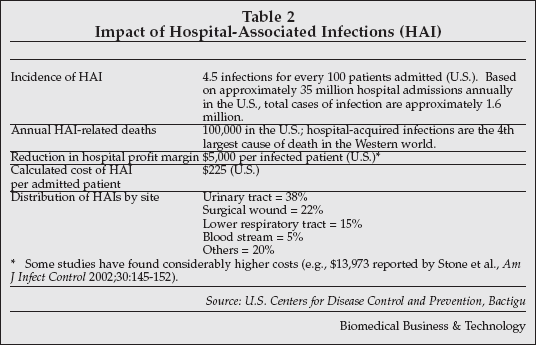 |
Bactiguard (Stockholm, Sweden) has developed the Bactiguard Infection Protection (BIP) Foley catheter, a device coated with a thin noble metal alloy consisting of silver, gold and palladium. The coating prevents bacteria from adhering to the surface of the catheter.
Studies in 80,000 patients have shown a 30%-40% reduction in urinary tract infections, the most common type of HAI, when Bactiguard coated devices are used. Catheters employing the Bactiguard coating have been marketed by C.R. Bard (Murray Hill, New Jersey) and used by more than 60 million patients. Bactiguard is now introducing the BIP as a generic infection-prevention Foley catheter in Europe, Asia and South America.
Another supplier of catheters with anti-microbial characteristics, Sungwon Medical (Choongchungbuk-do, Korea), exhibited the Prime-S catheter, a central venous catheter which employs a polyurethane coating containing silver.
Coat it — with silver
BioCote (West Midlands, UK) has taken the anti-infective coating concept a step further with the development of the BioCote coating that can be used on essentially any device as well as a wide range of surfaces throughout the hospital to provide protection against infection. The silver coating can be applied to standard hospital equipment at the point of manufacture, providing lasting antimicrobial protection.
At MEDICA, BioCote reported the results of a study performed at the Heartlands NHS Hospital in the U.K. comparing two out-patient wards, one containing furniture and equipment with BioCote antimicrobial protection and another containing standard, untreated items.
The items coated in the trial included door handles, light switches, tiles, blinds, and sack holders. The study found a 95.8% reduction in bacteria in the ward containing BioCote-treated items compared to the standard ward, and a 92.6% reduction in bacteria on the surfaces of BioCote-protected items. There was also a 43.5% reduction in bacteria on the surface of non-protected items that were located alongside items protected with BioCote. The effectiveness of the coating has been demonstrated against a number of organisms including MRSA, Legionella, Listeria, E-coli and Salmonella.
An alternative anti-infection coating technology, employing a copper oxide compound as the active agent rather than silver, was introduced at MEDICA by Cupron (Beit Shemesh, Israel). The Cupron coating can be applied to a wide range of textiles, non-woven fabrics, paper, latex, and other polymeric materials.
The anti-microbial activity of the coating typically lasts for the life of the product that is coated, and the coating has been demonstrated to be safe for human use. Laboratory studies have demonstrated a reduction of more than three logs in growth of odor- and stain-causing microorganisms.
Antimicrobial across the spectrum
GAMA Healthcare (London, UK) introduced a new medical wipe at the MEDICA exhibition that exhibits broad spectrum antimicrobial activity including activity against spores, typically a difficult class of organisms to eradicate with agents that are hand-safe. GAMA has developed a unique bilayer wipe containing a powder that becomes activated upon wetting to produce paracetic acid, which is both effective against spores and hand-safe.
The product is part of the company's Clinell line of medical wipes. The wipe's anti-microbial activity lasts for 24 hours following activation. The new product is expected to be significantly more expensive compared to standard medical wipes. The medical wipes market is a small but rapidly growing segment of the healthcare decontamination products market, totaling about $40 million in 2006 in the U.S. and growing at about 14% annually.
The second most prevalent type of hospital-associated infection is surgical wound infections. New technologies for prevention of wound infections are consequently also in strong demand as hospitals seek to reduce the incidence of HAI. The prophylactic use of antibiotics for prevention of wound infections is losing favor worldwide because of the increasing prevalence of antibiotic-resistant organisms such as MRSA, which now infects between 5% and 15% of hospital patients in Europe.
Silver-containing antimicrobial wound dressings, which do not promote the spread of antibiotic resistant organisms and are effective in preventing infections even by such organisms, are one technology that is gaining in popularity for prevention of surgical wound infections.
Silver antimicrobial wound dressings now account for about 12% of the advanced wound dressing products segment, which in turn accounts for about half of the total wound care products market.The silver antimicrobial dressing segment is growing almost twice as rapidly as the total wound care market and is led by companies including ConvaTec (Princeton, New Jersey), Smith & Nephew (London, UK) and Eth-icon/Johnson & Johnson (Warren, New Jersey).
Other technologies are vying for a position in the market, however.
For example, AM Scientifics (Geneva, Switzerland) exhibited the JUC Invisible Physical Antimicrobial Dressing at MEDICA, a spray-on dressing that creates a positively charged film on the skin. Since 99% of microorganisms are negatively charged, the film effectively captures and neutralizes invading bacteria, preventing active organisms from reaching the wound. The film remains active for eight hours when used on skin and can also be used on fabrics such as wound coverings, where it remains active longer.
Managing the wound
Advanced approaches to wound management are providing new options for hospitals to further reduce morbidity and adverse events.
SewonCellontech (Seoul, Korea) exhibited the Regeneration Medical System, a skin regeneration technology used currently in cosmetic treatments. In its current form, the Regen PRP system employs a patient-derived fibrin mesh as a matrix that is seeded with autologous platelets as a wound healing preparation.
The company is developing an advanced version of the technology for medical applications that uses the patient-derived fibrin mesh as a matrix for growth of other types of autologous cells such as keratinocytes, adipocytes, or bone marrow cells.
Kits are in development for launch by the end of 2007 for applications including skin regeneration (Regen Keratinocytes), adipose tissue regeneration (Regen Adipocytes), and other tissues (RegenExtraCell). The skin regeneration kit has potential applications in acceleration of wound healing.
In a pilot study of 60 patients with severe skin lesions, one group was treated with the Regen Keratinocyte system, a second group with the Regen PRP system, and a third group served as a control. The keratinocyte-treated group had the shortest healing time, five days vs. about seven and one-half days for the platelet-treated group and 12 days for the controls.
Another approach to reducing surgical site infections that is being adopted in an increasing number of hospitals worldwide is warming of patients during surgical procedures. Very strong evidence exists supporting the benefits of patient warming during surgery in reducing surgical site infections. A substantial market has developed, exceeding $100 million in the U.S. in 2006 as shown in Table 3, for products used to actively warm surgical patients.
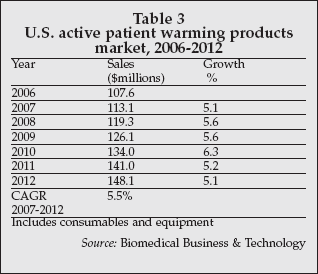 |
The leading supplier of active warming products worldwide, Arizant Healthcare (Eden Prairie, Minnesota), has achieved annual revenue growth approaching 10% over the past five years. In Europe, Geratherm Medical (Geschwenda, Germany) is a growing supplier of patient warming systems, addressing not only applications in reducing risks from wound infection but also in rescue of patients from hypothermia.
At MEDICA, Geratherm exhibited its PatientWarmingSystems line, employing carbon fiber technology developed in aerospace research. The carbon fiber system provides improved temperature control compared to chemical warming blankets, according to Geratherm, and eliminates the risk of burns associated with some other types of warming systems. The system also provides rapid stabilization at the preset warming temperature, and provides only two temperature setpoints, 37 C and 42 C, to simplify operation in emergency rescue operations, and can be operated from a variety of battery sources or mains power, increasing flexibility.
The Geratherm system employs reusable covers rather than disposable blankets like those used with the Arizant system. Over 3,000 Geratherm systems are in use worldwide.
