By LARRY HAIMOVITCH
Cardiovascular Device Update Contributing Editor
And Don Long, CDU Executive Editor
DENVER — The 28th annual scientific sessions of the Heart Rhythm Society (HRS; Washington) in early May, drew record attendance as interventional electrophysiologists (EPs) from around the world gathered to hear the latest information on a wide variety of topics relevant to their rapidly growing subspecialty. Overall, the conference is focused on pacing of the heart, maintaining its normal rhythms with a variety of pharmaceutical, procedural and device-related approaches. An even tighter focus is on the electrical activity of the heart and how it is managed, altered and restarted, if stopped.
One of the features of this activity is irregular rhythm, a speeding up that can be triggered by any number of things. One of the most common of these problems is atrial fibrillation (AF), which over the past few years has emerged as a major issue at HRS sessions and for very good reasons. AF is a disease with large prevalence and so a huge patient pool.
Less than one year ago, the prevalence of U.S. patients was generally agreed to be about 2.2 million persons. However, an article in the July 11, 2006 issue of Circulation, titled "Secular Trends in Incidence of Atrial Fibrillation in Olmsted County, Minnesota, 1980 to 2000, and Implications on the Projections for Future Prevalence," revealed that AF has been dramatically underestimated. The authors estimated that the prevalence exceeds 5.1 million. Moreover, by 2050 with the aging "Baby Boomer" population, it is expected to soar to nearly 16 million.
In addition to its massive prevalence, the current treatment modalities are proving to be less than ideal. For example, medical management with anti-arrhythmic drugs is sub-optimal, with an efficacy rate in the 50%-60% range and a high side effect profile.
Raising the risks
According to many studies, AF increases the risk of an ischemic stroke five-fold. To prevent an ischemic stroke, AF patients are often prescribed the blood thinner Coumadin. Owing to its very narrow therapeutic range, onerous adverse effects such as bleeding complications and inconvenient dietary interactions, the compliance rate for patients taking Coumadin is abysmally low, somewhere between 17% to 55%, depending on the study.
Percutaneous catheter ablation, which has yet to receive FDA approval and is performed by EPs using catheters approved for other cardiac arrhythmias, has shown tremendous growth in the past several years. At the 12th annual Boston Atrial Fibrillation Symposium held in January, it was estimated by several industry pundits that 30,000 to 35,000 "off-label" AFib catheter ablations were performed in the U.S. in 2006. Moreover, this number has soared in recent years, in response to the growing number of AF patients and the poor results from medical management.
This surprisingly large number of procedures has occurred despite mediocre clinical results. There is no agreement on what the level of catheter ablation efficacy is — mainly due what to defines a clinical "success" of an ablation procedure. In addition, since AF is a very complex disease with degrees of severity, treatment success will vary. The issue of success is complicated further by the different diagnostic monitoring follow-up techniques used.
'Success' not so easily defined
An article titled "Circumferential Pulmonary-Vein Ablation for Chronic Atrial Fibrillation" in the March 2, 2006, issue of The New England Journal of Medicine provided a good example of the loose definition of "success." Reporting on a randomized, controlled trial of AF catheter ablation vs the well-known anti-arrhythmic drug amiodarone, the authors indicated that they achieved a remarkable 74% "success" rate with catheter ablation treating the most difficult type of AF — the chronic or longstanding persistent variety.
Although the patients were given event monitors for one year, they were asked to record their rhythm for five days a week for just three minutes per day. This minimal amount of monitoring is generally regarded as completely inadequate for determining the success of an AF intervention. The recently-released HRS "Expert Consensus Statement Surgical Ablation of Atrial Fibrillation" recommended that 24-hour Holter monitoring be employed as a "minimal monitoring strategy" for patients enrolled in a clinical trial and recommended Holter monitoring at three to six month intervals for one or two years.
It is interesting to speculate on what the success of the above-mentioned trial might have been with more rigorous monitoring but it is safe to say that it would have been lower.
At this meeting, Hugh Calkins, MD, a prominent EP physician and head of the EP lab at the Johns Hopkins Hospital (Baltimore, Maryland) cited a meta-analysis of 21 trials of more than 50 patients treated with catheter ablation between 2003 and 2006. He said that the success rate in these trials varied between 40% and 80% and that multiple treatments could boost the success level to between 70% and 80%.
Commenting on this data, Calkins said, "We really do not cure atrial fibrillation, we treat it."
Further supporting the case that AF ablations are not very successful, an article in the April 2006 issue of the Journal of Interventional Cardiac Electrophysiology reported that the long-term, single-procedure efficacy of catheter ablation of AF is 28%. Moreover, a major complication occurred in 8% of the patients treated.
Given the prevalence of AF and the mediocre success of treatment, it is no surprise that for the first time ever, the HRS meeting dedicated two full days to AF. Dubbed the AFib Summit, this program covered a broad range of topics relevant to AF treatment modalities. And a significant number of presentations targeted attempts to improve the modest success rate. Several innovative technologies, aiming to improve the ease, safety and efficacy of the procedure, were discussed.
Improving the EP approach
Publicly-owned Stereotaxis (St. Louis, Missouri) has pioneered in this area with its proprietary system designed to provide greater efficacy, efficiency, predictability, ease of use and safety for routine and complex procedures in electrophysiology.
The four key components of its FDA-approved technology are:
- A magnetic navigation system that provides precise navigation and control of the working end of its disposable line of catheters and guidewires.
- Proprietary magnetically-enabled catheters, guidewires and other disposables devices that are used to deliver therapy.
- A sophisticated user-friendly interface and proprietary software user interface, through which the physician conducts the procedure from the control room adjacent to the cath lab, outside the x-ray field. This user interface is digitally integrated with the other key cath lab imaging technologies.
- Proprietary software to facilitate specific complex procedure types and protocols.
Despite the fact that this technology is expensive, with an average selling price of about $1 million, as well as significant additional construction costs to accommodate the system's large magnets and provide appropriate shielding, the company has achieved impressive growth in the past two years. Clearly, the "early adopters" in the EP community have responded to its attractive technology features.
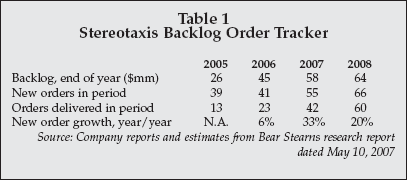
Table 1 shows the company's strong growth in the past two years and projections for the 2007-2008 period.
 |
A newer player in this market space is Hansen Medical (Palo Alto, California), which completed its initial public offering in November 2006. At the HRS meeting, Hansen bannered the FDA clearance of its robotic system, the Sensei, and the Artisan control catheter, designed for the accurate positioning, manipulation and stable control of catheters and catheter-based technologies.
Frederic Moll, MD, founder/CEO of Hansen, told Cardiovascular Device Update that the current gold standard "is manual control of catheters and because catheters are flexible tools it can be very difficult to control." The Sensei, he said, "is a way to transform a catheter into a surgical tool."
The system allows physicians to instinctively and remotely navigate catheters with greater stability and control in interventional procedures, with the goal of enabling physicians to perform procedures that historically have been too difficult or time-consuming to accomplish routinely with existing catheters and catheter-based technologies, or that could be accomplished only by the most skilled physicians.
In contrast to Stereotaxis, which has several FDA approvals for its system and been selling its system for about three years, Hansen recently received two key clearances. In May, it gained CE marking for the Sensei Robotic Catheter System and Artisan Control Catheter, which will enable MDs to perform interventional procedures. And it gained FDA clearance to commercialize these two systems to facilitate manipulation, positioning and control of mapping catheters during electrophysiology procedures.
The company's technology is not approved specifically for AF ablation, as there are currently no catheters expressly approved for this use in the U.S.
In April, the company reported that it had shippped two of its Sensei systems to its European sales distributor, and has shipped another system to a "Center of Excellence" at St. Mary's Hospital (London). In the U.S., Hansen has yet to make a commercial placement but has indicated that it expects to place several systems this year at an average selling price in excess of $600,000.
Although the goal of these competing systems is similar, i.e., precise catheter navigation, hopefully leading to better clinical outcomes, there are significant differences.
Whereas the Stereotaxis system relies on a magnetic field to enable the physician to remotely direct the catheter to different positions, Hansen's Sensei utilizes a robotic sheath and pulling wires to enable the physician to navigate the catheter to the intended spot while working from a computer-controlled, remote console.
There are also differences in the size of the systems and how they are installed.
With the Stereotaxis system, the EP lab must be designed around the system to house the large magnets. The room must be shielded properly and also has to be MRI-friendly. Thus, due to the large magnets and required room modifications, the Stereotaxis system is significantly more costly to purchase and install.
In contrast, the Hansen system can be used in any unmodified EP lab. The system has an open platform for disposables — the physician can use any EP catheter with this system whereas the Stereotaxis system requires specially designed, magnetic-tipped catheters.
The Stereotaxis catheters may be more attractive to some clinicians, as they do not need to be as stiff and pushable as standard EP catheters. As a result, there is minimal risk for vessel perforation with the Stereotaxis system. With the Hansen system there is a slightly higher potential perforation risk.
The Stereotaxis system also has broader applications. It can be used not only for EP applications such as AF ablation, but also for neurology and, peripheral vascular applications, biventricular pacing lead placement and ventricular tachycardia ablation. In comparison, the current Hansen system is designed primarily for atrial mapping and ablation.
Another robotically-guided catheter system is being developed by a newly-formed privately-owned company Catheter Robotics (CRI; Mt. Olive, New Jersey). Like the Hansen system, the CRI system uses any off-the-shelf catheter and can be used with any mapping system. Although the CRI is still in development, with regulatory hurdles yet to be addressed, it is expected to be priced well below the price point of both Stereotaxis and Hansen.
According to one of the world's experts on catheter ablation, Andrea Natale, MD, medical director for the Center for Atrial Fibrillation at the Cleveland Clinic Foundation (Cleveland, Ohio), robotically-controlled catheter ablation "represents the future of the EP lab because it can provide precise catheter navigation, enable operators to reach difficult areas and improve catheter stability as well."
Natale added that "catheter instability is one of the reasons for the long term failure of AF ablation because the physician is unable to keep the catheter stable for long enough to create a permanent lesion."
Advances — with limitations
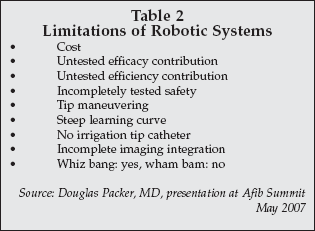
At the AFib Summit, Douglas Packer, MD, a prominent EP from the Mayo Clinic (Rochester, Minnesota) and one of the chairs of this meeting, discussed this technology with a talk titled "Image-Guided Robotic AFib." He noted that while there is great promise with this approach, there are several key limitations as well, as noted in Table 2. He described this new technology as "whiz bang: yes, wham bam: no."
 |
Another intriguing potential new technology to treat AF is being developed by privately-owned CyberHeart (Menlo Park, California). This non-invasive robotic stereotactic radiosurgery system technology, called the CyberKnife, was originally brought to market by publicly-owned Accuray (Sunnyvale, California) for the non-invasive treatment of various body tumors. It has been tremendously successful in that realm, as shown by the meteoric revenue growth and unit placement growth of Accuray in recent years.
Combining continuous image-guidance technology with a compact linear accelerator, this system has the flexibility to move in three dimensions according to the treatment plan, the CyberKnife System can autonomously track, detect and correct for tumor and patient movement in real-time during the procedure even in moving organs, enabling delivery of precise, high dose radiation typically with sub-millimeter accuracy.
Just prior to the HRS meeting, Accuray reported entering into an agreement with CyberHeart whereby the latter will modify Accuray's technology to develop a non-invasive method for performing cardiac ablation. In the event CyberHeart is able to successfully develop and commercialize such a system, Accuray will be the sole supplier of radiosurgery equipment to CyberHeart and will also be entitled to receive specified payments based on usage of the CyberHeart system.
CyberHeart recently indicated that it hopes to begin its initial clinical trials in 12 to18 months and to bring the product to market in the next three years. The company is currently in the midst of its first venture capital financing, which it hopes to close shortly.
At the HRS meeting, an abstract titled "New Non-invasive Therapy for Cardiac Arrhythmias using Stereotactic Radiosurgery: Initial Feasibility Testing" was presented.
The authors reported on treating five swine with the CyberKnife system and reported that it produced focused electrophysiologic changes with minimal changes in surrounding tissue. They concluded by saying that "this new methodology is potentially applicable to any cardiac arrhythmia."
Other approaches
Another approach to treating AF is through a surgical ablation. The goal of surgical ablation is identical to catheter ablation, i.e., to destroy the atrial tissue that is causing the chaotic electrical activity in the atrium. After an ablation, these aberrant electrical impulses cannot cross the burn scars that separate the areas of the atria, thereby halting the AF.
Although the surgical ablation of AF is not the domain of the EP and is less appreciated as a means to treat this problem, there is growing interest in this approach. Like catheter ablation, surgical ablation has grown significantly and reached an estimated 22,000 procedures in the U.S. in 2006. About 20,000 of these procedures are performed in conjunction with an open heart surgery procedure (typically for a bypass graft or valve replacement), with the remaining 2,000 being performed on a standalone basis.
The recent AF Consensus statement was supportive of surgical ablation and indicated that there are three appropriate indications.
- Symptomatic AF patients undergoing other cardiac surgical procedures.
- Selected asymptomatic AF patients undergoing cardiac surgery in whom the ablation can be performed with minimal risk,
- Stand-alone AF surgery should be considered for symptomatic patients who prefer a surgical approach, have failed one or more attempts at catheter ablation, or are not candidates for catheter ablation.
As evidence of the interest of EPs in surgical ablation, Atricure (West Chester, Ohio) sponsored a lunch-time seminar titled "AFib Ablation: What EPs Can Learn from Surgeons" that drew an overflowing crowd of EP doctors.
One of the keys to gaining a stronger foothold in the AF market will be to increase the referrals from EPs and cardiologists, who typically manage the AF patient. In turn, solid clinical data must be generated for these constituents to be confident to refer their patients.
Evidence of that was presented by cardiac surgeon James Edgerton, MD, from the Texas Hospital of the Southwest (Plano, Texas), who has reported perhaps the most impressive surgical ablation clinical data. Based upon an extremely rigorous definition of success — freedom from any episode of AF lasting three seconds or longer with a long term event recorder — Edgerton reported an 80% or higher success rate with his cohort of AFib patients who were treated on a standalone basis. He used the Atricure bi-polar radiofrequency ablation equipment for his cases.
Can EPs and surgeons work together to treat atrial fibrillation?
According to Josep Brugada, MD from the University of Barcelona Hospital Clinic (Barcelona, Spain) and co-chair of the Consensus statement, "I am absolutely convinced that EPs and surgeons can work co-operatively treating atrial fibrillation."
Quelling ICD concerns
Another problem of obvious concern to the attendees — seen more often in poster presentations than in the research discussions — is the small risk of failures by implantable cardioverter defibrillator (ICD) devices.
Those failures produced some of the biggest device headlines in 2005 and 2006 with large recalls of the devices. Still to be determined is the legal fallout from those recalls: how courts will rule on roughly 1,000 lawsuits, primarily against Guidant (Indianapolis), now the cardiac rhythm management (CRM) business of Boston Scientific (Natick, Massachusetts).
With launch of the conference, HRS and the American College of Cardiology (Washington) reported the release of the National Implantable Cardioverter Defibrillator (ICD) Registry, calling it the nation's first comprehensive database of detailed information about patients receiving these devices, an initiative launched in 2004 by the HRS and then involving, it said, up to 17 organizations.
HRS and the ACC said that the initial ICD Registry includes data provided by 1,450 hospitals, related to nearly 3,900 physicians and for more than 100,000 patients in its first year. The hope is that the registry will provide an improved guide for the use of these devices in patients currently implanted with them and those implanted in the future.
Laurent Lewkowieez, MD, director of clinical cardiac electrophysiology at the University of Colorado Hospital (Denver), called the registry data "invaluable … By analyzing patient characteristics and device trends over time, we can determine if changes need to be made to our medical protocols to ensure the best patient care possible."
Stephen Hammill, MD, chair of the ICD Registry steering committee, and a past president of HRS, said in a statement that a key benefit will be in enabling physicians to understand device performance "relative to their peers" and thus improve the quality of care.
However, in an interview with CDU, he modified that statement a bit.
A hospital will see data for their individual physicians but not from the physicians at other hospitals, he said. Individual hospitals also will be able to compare their data to, first, other hospitals in their own size category and, second, to the data from the entire national universe of hospitals, but not to the performance of physicians at the other hospitals. Others that will receive reports from the registry are third-party payers and manufacturers of ICDs.
Hammill said that manufacturers won't be able to use comparisons between their own performance and that of another manufacture, for instance as a promotional tool. They will only see their data compared to the aggregated data of all manufacturers.
Hammill said that moves to create the registry preceded the national headlines concerning ICD failures but was officially launched in September 2004 and then given further boost by the Centers for Medicare and Medicaid Services which wanted more information about what it was paying for in its reimbursements of ICDs. The agency then adopted the registry system in 2005.
Hammill acknowledged initial pushback from some hospitals concerning the registry because of the costs involved in implementation, but those objections went away in the face of non-reimbursement from CMS for those not registry-compliant. Hammill noted that the hospitals were invited to voluntarily submit information on all their patients implanted with ICDs, not just Medicare patients, to develop the registry's "benchmark scores."
About 80% of the hospitals voluntarily supplied information from non-Medicare ICD implantees, he said, and about 46% of the data came from non-Medicare patients.
The data reviews 100 data points, ranging from highly critical aspects of care - such as the rate of infection — to simple reporting of patient ages. To provide clarity to the 100 points of data, the report provides an executive summary highlighting the 10 most critical points for benchmarking simplicity.
Researchers will also receive access to the information, and Hammill predicted the development of up to four abstracts concerning the registry at the next meeting of the American Heart Association.
The first registry provides data only concerning the circumstances of original implantation, but Hamill said that future reports will track follow-up data to provide trending information. The registry "will go forever," Hammill told CDU, adding the hope that this initiative will be added to by similar registries covering, for instance, lead implantation, pacemakers and cardiac ablation procedures. Hammill said he thought the main benefit of the registry is that it will report "what's happening in real life and real-life populations — of people who are often older, often sicker.
A poster presentation at the conference appeared to indicate that ICD reliability is improving.
Researchers at the Minneapolis Heart Institute eamined dates of implant and replacement, reason for replacement and cause of failure for more than 1,200 ICDs from nine centers in North America, the devices examined those made by Medtronic, Guidant and St. Jude Medical. The researchers concluded that there has been continuous improvement in longevity and reliability for ICDs implanted in 2004 to 2006, compared to those implanted from 2001 to 2003.
Robert Huaser, MD, lead author and a cardiologist at the Minneapolis Heart Institute, referred to a lot of concern recently" about the performance of these devices and said that the study "should reassure physicians and patients that these devices have improved dramatically in recnet years and are now safer than ever.
Specifically, the study found:
- implant time increased 26% for dual-chamber devices implanted in 2004;
- implant time for single-chamber devices in 2004-2006 was almost 6 years, a 36% improvement over 20011-2003.
- only nine IDCS experienced unexpected failures in 2004-2006, compared with 35 in 2001-2003;
- four devices delivered inappropriate shocks or contributed to other major adverse events in 2004-2006, compared to 10 such device-related events in 2001-2003.
