Cardiovascular Device Update, Contributing Editor
NEW ORLEANS — The annual meeting of the American College of Cardiology (ACC, Bethesda, MD), held here in late March, was most notable for new revelations regarding stent therapy, and in particular comparisons of stents to medical treatment. However, there were numerous important advances described in other segments of the cardiology device market that highlight not only the diversity of the field but also this potential for development of major new market opportunities.
At the show, a representative of General Electric identified three key areas for cardiovascular care being highlighted at the meeting: drug eluting stent (DES) technology, increased clinicial workflow and advanced imaging. While drug-eluting stent (DES) issues effect a relatively small number of patients, and workflow is primarily a concern for clinicians — and their accountants — the many advances in imaging technology clearly are at the heart of cardiovascular diagnostics and the best therapeutic choices for the majority of heart patients.
IVUS improves stent deployment
One imaging modality that is becoming more important due in part to safety issues that have emerged recently with DES devices is intravascular ultrasound (IVUS), which can serve as a tool to help optimize stent deployment, one of the major factors believed to play a role in stent thrombosis. Incomplete apposition of stent struts to the vessel wall is thought to increase the risk of thrombosis and reduce delivery of drug to the injured vascular tissue, thereby increasing the risk of restenosis.
IVUS is also being used increasingly to characterize vascular lesions prior to treatment in order to select the optimal therapy, such as determining optimum stent diameter and length. Use of IVUS integrated with conventional angiographic imaging provides a powerful tool for analyzing a lesion prior to intervention and for assessing the results of treatment, not only immediately post-procedure but also at longer-term follow-up where the 3D capability of IVUS provides a more reliable and accurate method of determining lesion characteristics.
In addition, IVUS has emerged as a potential tool for detection of vulnerable plaque, thus offering a potential role not only in determining the optimum treatment for a lesion but also in detecting additional vascular segments that may benefit from treatment.
Leading suppliers of IVUS equipment include Volcano (Rancho Cordova, California) Boston Scientific (Natick, Massachusetts), and, outside the U.S., Terumo Medical (Tokyo).
Just prior to the ACC conference, Boston Scientific reported that sales of its new iLAB Ultrasound Imaging System, which integrates IVUS directly into the cath lab and provides a tableside controller allowing control of IVUS imaging from the sterile field, had reached 300 units worldwide in nine months. That is double the number of placements of the company's prior-generation Galaxy 2 system.
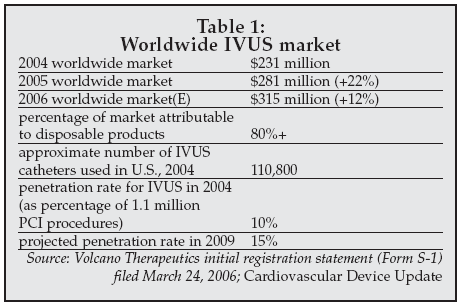
The rapid growth in placements is an indication of the increasing utilization of IVUS in intravascular imaging. Sales for Volcano have also increased substantially in recent years, driving substantial growth in the IVUS market as shown in Table 1.
 |
However, as discussed by a number of speakers at an ACC session on emerging imaging technologies, there is now growing evidence that multiple new intravascular imaging modalities, as well as some non-invasive imaging techniques, may also prove important for optimizing the ability to characterize CAD and select the best therapy.
OCT combined with IVUS
Additional intravascular imaging modalities that are showing promise include Optical Coherence Tomography (OCT), a high-resolution imaging technology under development by LightLab Imaging/Goodman (Westford, Massachusetts) and Cardio-Spectra (San Antonio); infrared spectroscopy, being developed by InfraRedx (Burlington, Masssachusets); and intravascular MRI, being developed by TopSpin Medical (Tel Aviv, Israel).
As discussed by Stephane Carlier, MD, PhD, of the Cardiovascular Research Foundation (New York) at the ACC sessions, IVUS is a powerful technique for plaque characterization and also possibly may have a role in prognosis of myocardial infarction, but does not perform well in calcified lesions. OCT, on the other hand, is well-suited to analysis of calcified plaque, so Carlier is investigating combination IVUS/OCT imaging.
Hiroyuki Ozaki of the Yokohama City University Medical Center (Yokohama, Japan) described the use of combined IVUS/OCT imaging for plaque imaging, showing examples of exams where OCT, with a 10-fold higher spatial resolution compared to IVUS, could detect plaque disruption, but could still not reliably detect vulnerable plaque. Combined IVUS/OCT imaging could, however, detect vulnerable plaque.
I-MRI added to mix
A similar approach was described by Ron Waksman, MD, of Washington Hospital Center (Washington) at the sessions, adding yet another technique, intravascular MRI (I-MRI), to the imaging armamentarium. I-MRI can differentiate lipid-rich vs. fibrous plaque, and a first-in-man study using the technique is now under way in the U.S. with about 25 patients enrolled so far.
The utility of OCT may be further enhanced by a variation of the technique being developed by Cardio-Spectra which employs frequency domain analysis of the optical signals rather than time domain analysis as is used in the LightLab device.
The Cardio-Spectra technology uses Fourier analysis to image inside blood vessels and avoids the need for balloon occlusion of the vessel in order to clear blood from the region to be examined. Instead, a saline flush can be used, improving the user-friendliness of the technique. Cardio-Spectra is now using its Fourier OCT imaging technology to perform molecular and cellular imaging.
In one study, the system has been shown to allow specific detection of macrophages, which are often concentrated in regions of vulnerable plaque, using Ultrasmall Super Paramagnetic Iron Oxide (USPIO) particles that bind to macrophages, and then applying an oscillating external magnetic field in the region being imaged to detect localized oscillations in the OCT signal.
Combining advanced OCT methods with technologies such as IVUS for plaque characterization could provide a powerful approach to determining the optimal treatment strategy for coronary artery disease.
In particular, with the recent revelations of equivalence in outcome for some groups of patients treated with medical vs. interventional therapy — seen in the results of the COURAGE trial that appeared to promote standard medical therapies over PCI/stenting — there may be an important role for technologies that allow improved discrimination of those patients who will benefit from PCI vs. those who can receive the same outcome with medical therapy.
Unlike IVUS, OCT has not yet been cleared by the FDA for clinical use, but at least one system has been submitted for FDA marketing clearance and is expected to be on the market sometime in 2007. When introduced, OCT systems are expected to be priced at around $100,000, with single-use catheters selling for about $1,000 each.
Infrared optical imaging systems, such as the one under development by InfraRedx, may also reach the market in the near future. InfraRedx is planning to submit an application for marketing clearance to the FDA sometime in 2007 for a device to detect lipid-rich plaque.
High sensitivity with CT
Non-invasive imaging of coronary artery disease is also continuing to advance. CT imaging using 64-slice scanners has now been shown to have a sensitivity of 98-99% for detection of a clinically important (50%) stenosis compared to conventional angiography. That level of sensitivity is adequate for clinical purposes according to experts presenting at the ACC sessions, and studies now indicate that at least 95% of at-risk patients can be identified using CT angiography.
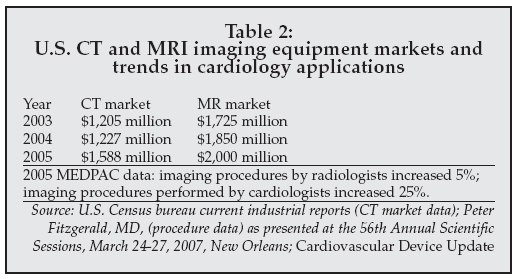
Toshiba (Tokyo, Japan), one of the leading suppliers of CT systems worldwide, is conducting a study using its 64-slice scanner with the SurePlaque feature to compare CT to conventional angiography in the CORE 64 trial, with over 400 patients recruited so far. The technology can also subclassify plaque as calcified or as two different types of soft plaque, potentially providing a significant added advantage compared to angiography. As a result, imaging procedures performed by cardiologists are increasing at a rapid rate, as indicated by the data in Table 2, which also depicts the growing market for CT scanners in the U.S.
 |
MRI offers challenge to stress testing
MRI technology is another non-invasive modality poised to play an increasingly important role in cardiology.
As discussed by Juerg Schwitter, MD, of University Hospital (Zurich, Switzerland) at the ACC sessions, MRI may be on the road to replacing nuclear imaging for cardiac stress testing. Use of gadolinium-based MR contrast agents to perform cardiac stress imaging has been shown to allow discrimination of scar tissue versus viable tissue in patients following a heart attack with equivalent discriminatory power as SPECT imaging, the standard technique now employed in cardiac stress imaging.
However, MRI can also detect tissue ischemia and now has sufficient spatial resolution to determine the degree of stenosis in a coronary artery, while avoiding the radiation exposure characteristic of techniques such as CT scans and SPECT imaging.
At present, the contrast agents used to perform cardiac MR stress imaging are investigational in the U.S., although they are in clinical use in Europe. Reimbursement is also an issue for clinical adoption of MR stress imaging, but one that Schwitter believes can be resolved given the advantages of the technique.
For example, in addition to avoiding radiation exposure, the time required for a cardiac MRI stress exam is 55 and 75 minutes, compared to four hours for a nuclear stress test, and MRI provides direct detection of scar tissue in the heart as well as reduced artifacts compared to nuclear imaging.
Although there are some issues with adverse tissue reactions to the gadolinium-based contrast agents used in MR stress imaging such as nephrogenic fibrosis, Schwitter quoted the rate of such events at 1 per million exams, and also noted that patients at elevated risk for such events can be readily identified and either imaged using reduced levels of contrast or referred to nuclear imaging.
Guiding the intervention
The use of imaging for guidance of cardiac interventions is another growing application in cardiology. CT is now being evaluated for use in guidance of cardiac ablation procedures, providing 3D images of the pulmonary veins and the region targeted for ablation to improve the ability to accurately target ablation.
Another development is robotic guidance of cardiac ablation procedures, using systems such as the Sensei Robotic Catheter System and the Artisan Control Catheter from Hansen Medical (Mountain View, CA). As described by Walid Saliba (Cleveland, Ohio) at the ACC sessions, the Hansen system is now being used along with targeted ablation devices such as the AcuNav intracardiac echocardiography catheter and the CARTO system from Biosense/Cordis to allow remote control of ablation procedures.
Saliba has demonstrated treatment success rates that are equivalent to those achieved with manual ablation, but with the advantages of providing access to regions that are difficult to reach manually and avoidance of radiation exposure for the operator.
Devicer opportunities for improved therapy
Advances in treatments for heart failure were described that are changing the way patients with that disease are managed, improving the efficiency of patient management for physicians and allowing adverse trends in patient condition to be detected earlier to enable interventions to be performed before a crisis develops.
Several device technologies described at the conference promise to improve the management of hypertension, as well as of cardiogenic shock and acute myocardial infarction.
Heart failure management continues to be a major focus for device manufacturers, with numerous new technologies under development for monitoring of treatment with devices such as implantable cardioverter defibrillators (ICDs), for heart assist in patients with failing hearts, and, possibly in the long term, for repair of damaged heart tissue.
ICD-based cardiac resynchronization therapy (CRT, combined with ICD, termed ICD-CRT) is now an established approach for management of certain populations of heart failure patients, and is becoming increasingly sophisticated due to advances in telemetry and miniaturization of implantable devices.
As discussed by William Stevenson, MD, of Brigham & Women's Hospital (Boston) at the ACC sessions, ICDs with remote monitoring capability can now detect dangerous arrhythmias such as sustained atrial fibrillation (AF) in heart failure patients, allowing device settings to be altered to help avoid such events.
Devices also are available or in development that allow parameters such as thoracic impedance, heart rate variability, activity level, respiration, blood pressure, body weight, and device function to be monitored remotely, in some cases without the need for patient interaction, allowing proactive management of heart failure to help prevent decompensation by detecting deteriorating trends and intervening earlier.
Key suppliers in the segment include Medtronic (Minneapolis) with the CareLink system; Boston Scientific/Guidant (Natick, Masssachusetts) with the Latitude system; Biotronik (Berlin) with its Home Monitoring system employing the CardioMessenger; and St. Jude Medical (St. Paul, Minnesota) with the Housecall Plus system.
Remote monitoring of ICD and ICD-CRT devices is becoming a key competitive factor in the cardiology market. As discussed by Stevenson, remote monitoring capability is rapidly becoming a necessary feature for ICDs. Currently available devices differ in their capabilities, as shown in Table 3
 |
Automatic transmission of data is generally a desirable feature, since patients must otherwise initiate a transmission which can be difficult to schedule and perform reliably, in some cases requiring up to 30 minutes of a patient's and a call nurse's time. There is some concern about liability of the physician if automatically transmitted alerts are missed and not acted upon, but so far that has not proven to be a significant problem, and the added efficiency afforded by remote monitoring outweighs issues with responding to alerts and managing the large volume of data received.
One factor is that alarm rates for automatic monitoring devices are very low. In Europe, for example, there were only 66 alarms generated over a one-year period in which 3 million ICDs were being monitored.
Physician, nurse and patient time savings can be substantial with remote monitoring. According to Stevenson, up to 30 patients can be managed with one-hour data analysis each morning using remote monitoring, whereas an in-clinic visit can take an entire day. However, physicians have not yet devised optimal methods to deal with the large amount of data that comes in, and so may need to establish data archives and methods to extract data in a prioritized fashion in order to realize the savings in efficiency and improvement in care that are possible.
Reimbursement for remote monitoring is no longer an issue since the passage of new rules by the Centers for Medicare and Medicaid Services last summer authorizing payment for patient check-ups via remote monitoring. In some states (for instance, Illinois), there is no limit on the number of check-ups that will be paid for, but generally monthly checks are allowed. Costs for remote monitoring, other than for nurse and physician time, vary, depending on the system.
But cost issues still abound
For the Guidant/Boston Scientific Latitude, for example, there is no added charge for the life of the implant, but the initial cost of the device including the remote monitoring service is 10%-12% higher. Biotronik absorbs all costs for its remote monitoring network. Medtronic charges a fee for its CareLink remote monitoring service, but the cost is dropping according to Stevenson.
To date, manufacturers have had difficulty justifying any added cost for remote monitoring capabilities since there are no randomized trials demonstrating cost savings due to improved efficiency, or demonstrating improved patient outcome due to better disease management. However, that may change in the near future, according to Stevenson, since results of studies showing improved patient outcomes and cost savings are about to be published.
Manufacturers are continuing to enhance device and system capabilities. For example, Medtronic demonstrated new long-range versions of its wireless ICDs which allow the patient to be up to five meters away from the base station in a clinic setting for transmission, and even further away in the home setting. Medtronic now has more than 110,000 patients being monitored on its CareLink network in the U.S. alone.
Supporting the heart mechanically
Mechanical therapies also are expected to play an important role in heart failure therapy, particularly in the early stages following a heart attack where recent studies have shown benefit, as well as in late-stage patients either as a bridge to transplant or for long-term mechanical support.
Based on data presented at the ACC sessions, a new version of the Impella mechanical assist device from Abiomed (Danvers, Massachusetts), could prove to be an important advance for patients with cardiogenic shock. The Impella Recover LP2.5 has been evaluated in a study described by Melchior Seyfarth, MD, of the Technical University of Munich (Munich, Germany) comparing patients treated with the Impella to a group treated with intra-aortic balloon pump therapy in the ISAR-SHOCK trial.
There is a 6%-8% incidence of cardiogenic shock in myocardial infarction patients, with an associated mortality rate of about 50%. Use of mechanical support to restore hemodynamic flow in such patients can potentially avoid the extensive ischemic damage to heart tissue that occurs in such patients.
In the study, patients treated with the Impella device had a higher cardiac index at follow-up, and cardiac blood flow was substantially improved — by 1.1 liters/minute with Impella vs. 0.2 liters/minute with IABP therapy.
Seyfarth said that the Impella device can be used in 90% of patient with cardiogenic shock, and there were fewer complications with Impella compared to IABP patients. The Impella Recover LP 2.5 is on the market in Europe and is under investigational status in the U.S.
A unique new treatment for hypertension has been developed by CVRx (Maple Grove, Minnesota) called the Rheos Baroreflex Hypertension Therapy System, and was discussed at an ACC session on emerging therapies. The Rheos system employs electrostimulation technology and consists of an implantable pulse generator and electrodes that deliver energy directly to the carotid arteries. The principle on which the device is based is artificial control of the natural baroreflex via electrical stimulation, an approach that has been shown to reduce blood pressure in animals in extreme cases.
The Rheos system has been evaluated in the BaroReflex Activating System Study (BRASS), completed in 2003, which showed a linear reduction of blood pressure with applied voltage, and no adverse tissue reactions to the implanted device or electrodes. Systolic blood pressure was reduced from 177 to 167 mm Hg in the study, and a reduction in diastolic pressure was also observed. CVRx is now starting a 300-patient Phase III pivotal trial in the U.S., targeting patients whose hypertension cannot be adequately controlled with medical therapy.
Cell-based therapies keep coming
Additional advances in heart failure management were presented at the ACC sessions in the area of cell-based therapy. Cell therapy has been under study by a number of groups for treatment heart failure, and has generally been shown to be safe in pilot feasibility studies, with some indications of efficacy.
Joshua Hare, MD, and a group from the University of Miami School of Medicine (Miami, Florida) described a Phase 1 study with intravenous adult human mesenchymal stem cells for treatment of heart tissue damaged as a result of myocardial infarction. The study employed a preparation of allogeneic stem cells called Provacel derived from bone marrow using a process developed by Osiris Therapeutics (Baltimore, Maryland). A total of 53 patients were treated who had a first myocardial infarction between one and 10 days prior to randomization. Adverse event rates were equivalent in patients treated with Provacel vs. controls, and rates of re-hospitalization were statistically equivalent at 23.5% for treated patients vs. 37.6% in controls.
A significant decrease in arrhythmia was observed (8% in treated patients vs. 32% in controls), and there was also a lower rate of premature ventricular contractions observed in the treated group of patients. Pulmonary function and global cardiovascular function also improved in the treated patients. The fact that there was no adverse immune reaction or excess arrhythmias in the cell therapy population is a promising development, since that has been a drawback of cell therapy in prior studies. Cells were delivered via both intravenous and intramuscular routes.
A second study, described by Nabil Dib, MD, of the University of California at San Diego, employed autologous skeletal myoblasts for the treatment of ischemic cardiomyopathy. The trial, called CAuSMIC, for Catheter-Based Delivery of Autologous Skeletal Myoblasts for Ischemic Cardiomyopathy, was sponsored by Mytogen (Charlestown, Massaschusetts/Phoenix, Arizona).
Mytogen was founded as Diacrin in 1990 and subsequently acquired the intellectual property for skeletal myoblast transplantation from Genvec (Gaithersburg, Maryland), when that company decided to exit the field to focus on development of drugs for cancer and infectious disease therapy. Dib, as well as John Hodgson, MD, of Phoenix, are key principals in the company.
Delivery of the cells was guided using the NOGA Cardiac Navigation System and the MYOSTAR catheter from Cordis/Johnson & Johnson (Miami Lakes, Florida). using a percutaneous needle which penetrated from 2 mm to 10 mm. The patients all had Class II-IV heart failure, and ICD's were not implanted as part of the protocol, although some patients already had devices in place.
Study results demonstrated a dramatic improvement in functional class at one month follow-up, with 83% of the treated patients showing improved ejection fraction as assessed by echocardiography and 17% showing no improvement. In contrast, none of the control patients showed any improvement, and 36% had a decline in functional class.
A comparison of the effects of various medical and interventional treatments observed in other trials showed the degree of improvement — 1.4 on the New York Heart Association scale — to be greater than that for CRT, ACE inhibitors, or beta-blocker therapy. The company has now received FDA clearance for an expanded 160-patient Phase II clinical study.
Adult stem cell use
Another company developing cell-based therapies for cardiovascular disease, Baxter Healthcare (Deerfield, Illinois), prior to the ACC conference reported initiation of a Phase II trial using adult stem cells in the treatment of chronic myocardial ischemia. Promising results were obtained in a Phase 1 study completed last September.
The therapy involves using the company's Isolex instrument to capture CD34-positive adult stem cells from patients' blood, and then using a catheter-based system to deliver the autologous cells to regions of the heart with poor blood flow. Patients being treated in the study are those with severe CAD and not candidates for treatment with other options such as medical therapy, coronary stents/angioplasty, or coronary artery bypass graft surgery.
Another application of cell therapy was described at the conference by Kennyata Cosby of Johns Hopkins University (Baltimore). Cosby and others, including Dorota Kedziorek of the Institute for Cell Engineering (Johns Hopkins; Baltimore, Maryland), have developed a system for cell therapy delivery that can be visualized via X-ray, and they are focusing on use of the system to treat peripheral artery disease. The system, at present in the pre-clinical development phase, uses a microencapsulation technique employing a nanolayer alginate coating to accomplish direct labeling of stem cells and implantation in regions of ischemic tissue.
The researchers have also evaluated tagging of stem cells using magnetic labels such as Feridex for MRI guidance, but, according to Cosby, such labels can affect cell differentiation. The new approach could address some of the 5 million patients in the U.S. with intermittent claudication; 20% have critical limb ischemia and are often not candidates for revascularization therapy.
