CDU Contributing Editor
CHICAGO — The diagnosis and treatment of heart disease is continually being transformed by the application of new technologies. Diagnosis of heart arrhythmias, for example, has migrated from static electrocardiography performed in a hospital or clinic setting to ambulatory Holter monitoring and most recently to wireless remote monitoring of arrhythmia events as well as remote monitoring of patients treated with devices such as implantable cardioverter defibrillators (ICDs). Treatment of arrhythmias also has advanced due to innovations in implantable devices as well as in interventional technologies for ablation of arrhythmia-generating aberrant pathways in the heart's neural network.
For heart failure (HF), advances in non-invasive measurement technology as well as in new biomarkers are enabling improved diagnosis, and remote monitoring technologies are helping physicians manage heart failure patients outside the hospital to reduce re-hospitalizations.
Variety, breadth of offerings
The latest advances in all of those areas were presented during the annual Scientific Sessions of the American Heart Association (AHA; Dallas), held at the lakeside McCormick Place in mid-November. New developments in tissue engineering also were described that may eventually revolutionize HF treatment, as well as the treatment of heart valve and peripheral vascular disease.
While not all of the new technologies described here will succeed in the marketplace, the breadth of new developments demonstrates the continued investment in the cardiovascular device segment by existing suppliers, as well as by venture investors.
One factor that draws companies to the cardiovascular device segment is the large number of potential patients to be diagnosed and treated, numbering well over 100 million worldwide. As shown in Table 1, the total number of individuals with symptoms of either cardiovascular disease or coronary heart disease in the U.S. and Europe combined was about 120 million in 2004, and historical trends indicate that the size of the target population will expand significantly in the future.
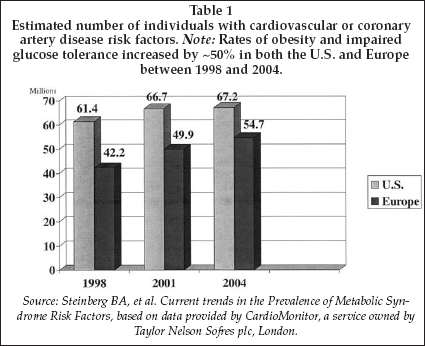 |
Symptoms of patients who were classified as having disease in the survey performed to generate the estimates shown in the table included hypertension, ischemic heart disease, angina pectoris, congestive heart failure, atrial fibrillation and other arrhythmias, cerebral ischemia, peripheral arterial disease, dyslipidemia, Type 2 diabetes, metabolic syndrome and other cardiovascular diseases as specified by a physician.
Advances in screening
Screening of the large at-risk population, which in the U.S. accounts for almost one-quarter of the total population, in order to identify those individuals who can benefit from treatment remains a challenge due to cost and compliance issues as well as lack of screening technologies with proven effectiveness.
One technology that has been advocated by many researchers for use as a general population screening tool to detect atherosclerosis is carotid intima-media thickness, or CIMT, which is measured by ultrasound imaging using systems such as the MyLab30CV, a $50,000 analyzer manufactured by Biosound Esaote (Genoa, Italy), along with the company's IMTLAB software. The MyLab30CV is one of a number of compact portable ultrasound imaging systems now on the market that are suited to use in a physician's office or clinic setting.
Other suppliers include Sonosite (Bothell, Washington), GE Healthcare (Waukesha, Wisconsin), Fukuda Denshi (Tokyo) and Mindray (Shenzhen, China). So far, IMT screening for atherosclerotic disease is not reimbursed by Medicare in the U.S., or by many private insurers. However, suppliers such as Biosound believe reimbursement could be approved soon as the benefits of early detection become widely recognized. At present, there is no active national coverage analysis in process at the Centers for Medicare & Medicaid Services (Baltimore) to analyze whether a change in reimbursement policy is warranted.
Assessing arterial stiffness
As discussed by Stephane Laurent of Assistance Publique-Hopitaux de Paris (an affiliate of Inserum), at an AHA symposium on non-invasive carotid imaging, the most effective screening technique may be a combination of IMT testing and analysis of pulse wave velocity (PWV), which is a measure of arterial stiffness. A system for PWV analysis of the carotid arteries, the ART.LAB, also is available from Biosound, although the ART.LAB is not currently available in the U.S.
Like IMT, vessel stiffness in the aorta also has been shown to be predictive for cardiovascular events, but there is significant variability in the readings from one person to another. Combining the two measures will provide a more accurate indication of cardiovascular risk, according to Laurent.
Another ultrasound imaging technique that shows promise for cardiovascular risk assessment was described at the AHA sessions by Flordeilza Villanueva, MD, of the University of Pittsburgh School of Medicine, that employs a microbubble-based ultrasound contrast agent to target regions of ischemia in the coronary vessels. The contrast agent consists of a dispersion of microbubbles about the size of red blood cells that can be surface-modified to attach various specific binding molecules, resulting in an agent that targets selected markers in the vessel wall.
Villanueva has synthesized a microbubble agent that specifically targets P-selectin, a marker that is indicative of tissue ischemia and that is expressed within minutes of ischemic reperfusion. The technique, ultrasound ischemic memory imaging, has been shown to detect the existence of prior ischemia as well as the size of the ischemic region, allowing non-invasive assessment of occlusive vascular events, even in cases in which biomarkers such as troponin, which measure tissue necrosis, are negative.
The agent could be useful in the chest pain clinic, according to Villanueva, for detection of myocardial infarction, and also could have applications in additional risk assessment of patients for myocardial infarction. The ischemic memory effect lasts at least up to an hour, based on Villanueva's studies, but the researchers believe it could persist for up to 48 hours.
Other applications now being investigated for the targeted microbubble agent using different selective binding molecules include detection of rejection of heart transplants; detection of angiogenesis (via labeling with vascular endothelial growth factor), which also could be an indicator of compromised blood flow in the coronary arteries; and assessment of vascular wall injury.
Heart disease biomarkers
The use of biomarkers for heart disease screening, as well as for diagnosis and therapy monitoring, was another key topic addressed at the AHA sessions. Brain natriuretic peptide, or BNP, is one important new marker that has emerged within the past four years as a significant aid in the diagnosis of heart failure. The test was initially introduced to the market by Biosite (San Diego) as a point-of-care assay and now also is available on automated immunoassay systems marketed by Beckman Coulter (Brea, California).
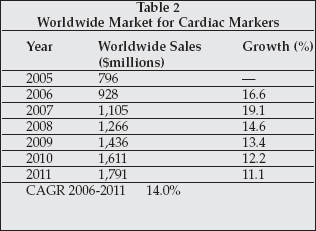
Another version of the BNP test, for N-Terminal proBNP, is marketed by Roche Diagnostics (Indianapolis), and has essentially equivalent clinical performance and utility. BNP is one of a small group of cardiac markers that has become widely adopted in the global market. As shown in Table 2, sales of all cardiac maker products combined totaled almost $800 million in 2005, and the market is expected to expand at a 14% compound annual rate through 2011.
 |
A study presented at the AHA sessions by Gordon Moe, MD, of St. Michael's Hospital (Toronto), the IMPROVE-CHF study, assessed the improvement in HF management in the hospital emergency department setting in Canada as a result of implementation of NT-proBNP testing. The study followed similar trials in the U.S. that had demonstrated a cost benefit, as well as improved outcomes, for patients managed using BNP testing. The IMROVE-CHF study sought to determine if similar benefits would be realized in a country with lower per capita income and a universal access, publicly funded health care system that mandates resource allocation.
The study results have implications for expanded use of BNP testing in management of heart failure patients in most developed countries outside the U.S., since they also operate nationalized, publicly funded healthcare systems. The results confirmed that clinical benefits in terms of reduced re-hospitalization rates were realized, and a cost savings of C$1,000 per patient was documented. Patients also had a shorter stay in the emergency department.
Other markers that are being evaluated that may further improve heart failure management, as discussed by David Feldman, MD, of The Ohio State University (Columbus), at the AHA sessions, include matrix metalloproteinase-9, high-sensitivity C-reactive protein, interleukin-6, 8-hydroxy-2-deoxyguanine, and ryanodine receptors. Feldman also discussed the use of one- and two-dimensional strain measurements performed via ultrasound imaging as a possible future diagnostic modality for heart failure. However, recent data indicates that BNP may provide a better correlation with response to therapy in heart failure compared to ultrasound parameters.
Targeting of anti-arrhythmia, HF treatments
Advances in electrocardiography-based testing for heart disease were also described at the AHA sessions. Microvolt T-Wave Alterans (MTWA), a technique pioneered by Cambridge Heart (Bedford, Massachusetts), was shown to provide equivalent capability as a predictor of sudden cardiac death compared to the more invasive electrophysiology catheter study that is typically used today. MTWA is already reimbursed by Medicare and some private insurers, including Wellpoint (Indianapolis), which an-nounced a coverage decision during the week of the AHA conference.
The ability to detect heart rhythm abnormalities indicative of a risk for sudden cardiac death (SCD) with more accuracy compared to existing techniques could drive growth in testing, particularly since MTWA is a non-invasive method. A study conducted by Ottorino Costantini, MD, of Case Western Reserve University (Cleveland), used MTWA to determine which patients would benefit from an ICD implant to prevent SCD, and found that the technology was as good as electrophysiology (EP) catheter analysis, and significantly better than the use of ejection fraction measurements commonly used today. Using ejection fraction to select patients for an ICD implant, 17 patients must be treated in order to save one life, whereas using EP studies for patient selection the number needed to treat to save one life is reduced to four.
EP studies are invasive, however, and also are expensive, costing between $1,000 and $2,000 per exam, according to Costantini. MTWA, on the other hand, provides equivalent selection efficacy non-invasively at a cost of about $250 per exam. Reducing the number of patients who needlessly receive ICD implants is important since the devices are costly (about $30,000 each for the device alone) and there is some risk associated with device failure. In addition, some patients who could benefit from an ICD implant are not receiving one because existing selection methods are inaccurate.
About 200,000 patients are expected to have an ICD implant in 2006, and the number is projected to rise 20% by 2008. Use of more accurate selection methods, which would combine MTWA and EP, may not affect the number of ICD implants significantly. While there are obviously many patients who are receiving unneeded implants at present, there also are some who are not being treated who could benefit, resulting in a minimal net impact on the implant rate.
Patient-driven demand
Another factor driving the market for ICD implants is patient demand. A study of patient perceptions of the survival benefit of ICDs for prevention of death from heart failure, presented at the AHA sessions by Garrick Stewart of Brigham and Women's Hospital (Boston), revealed that HF patients believe that an ICD saves about 50 lives per 100 implants, much higher than the seven to eight lives actually saved. That factor obviously biases patients in favor of having an implant, potentially overriding information from randomized trials that indicates a low probability that they will benefit based on their clinical characteristics.
New devices for monitoring of patients with heart arrhythmia were introduced at the AHA conference by a number of suppliers. Spacelabs Healthcare (Issaquah, Washington) exhibited the new EVO Holter System, a 3-lead ECG monitoring device that is about the size of an iPod Nano, and which includes a rapid-recharge battery that can be fully charged during the approximately five minutes required to download patient data at the end of a monitoring session. The product, which will be released shortly, will be priced at about $1,900. In addition, Spacelabs soon will introduce the Sentinel open architecture data management system that provides data analysis capabilities for Holter and stress ECG data.
Cardiomedics (Irvine, California) introduced the Carditrack, a 6-lead Holter device that derives a 12-lead ECG and employs wireless Bluetooth technology to transmit ECG data to a personal data assistant or preferably to a cellphone, from where the data can be uploaded to a monitoring center. The company has partnered with Motorola (Schaumburg, Illinois) to develop its Bluetooth technology. The system has received FDA marketing clearance, and will be available early this year. It consists of a disposable patch with integral ECG electrodes that transmit data wirelessly during a monitoring session, which is then forwarded via a cell phone to the Cardiomedics' monitoring center where a report is generated and published online within 20 minutes after completion of the 24-hour assessment period.
Up to five years of ECG data can be stored at the Cardiomedics center for reference. The company provides a disposable Biopatch and cellphone to the patient's physician at no cost, and the physician instructs the patient in use of the system.
Cardiomedics bills the patient's insurance for the $75 technical component, while the physician bills for the professional fee, resulting in an approximate 50/50 split of revenue. When used three times per week, Cardiomedics estimates physician revenue at about $16,000 per year. Physicians incur no capital or consumable costs, and use of a single electrode patch minimizes setup time.
Another company introducing new technology for 12-lead ECG monitoring, Signalife (Greenville, South Carolina), has introduced the Fidelity 100, a true 12-lead system that uses Bluetooth technology for interface of a patient ECG module to a central processing unit. The Fidelity 100 is designed for use in resting and exercise ECG testing, and incorporates a proprietary signal processing technology to significantly reduce artifacts.
In the DIVA study conducted at the Duke Clinical Research Institute (Durham, North Carolina) comparing the Fidelity 100 to the DR180 from NorthEast Monitoring (Maynard, Massachusetts), the Signalife system provided more accurate ECG data. Signalife's signal-processing technology avoids the need to filter the ECG signal, instead employing discrimination of artifacts at the front end. The existing system was introduced in May 2006, and is priced at $18,000. The next-generation product for Signalife in the ECG segment will be a Holter monitor for use in ambulatory monitoring that will allow remote ECG assessment using a small patient-worn device.
Progress in stem cell research
Another topic highlighted at the AHA sessions was the use of stem cells for heart repair
The use of stem cells harvested from adipose tissue isn't a new idea. But Paul DiMuzio, MD, associate professor of surgery at Thomas Jefferson University (Philadelphia), said in his presentation that this harvesting has been done from "young and healthy" patients, primarily as part of a cosmetic surgery procedure. In this study, it was determined that adipose stem cells can be collected from the elderly patients with cardiovascular disease — that is, those most likely to benefit from stem cell treatment for heart damage.
The researchers isolated stem cells from fat collected via standard liposuction from 49 patients undergoing cardiovascular procedures; the cells were then cultured for seven days. The resulting stem cells were determined to be healthy and viable and "not affected by the presence of associated disease, such as end-stage renal disease." In his presentation though, DiMuzio did qualify this by saying that the number of stem cells was not as great when taken from diabetic patients.
Thorsten Dill, MD, of the Department of Cardiology and Cardiac Imaging, Kerckhoff-Heart Center (Bad Nauheim, Germany), presented research demonstrating that bone marrow stem cells could be used to improve the repair of left ventricular strength and function after serious heart attacks, those described as ST-segment elevation of myocardial infarction.
MRI analysis demonstrated that the treatment with the bone marrow stem cells — via injection into the patients' coronary arteries — produced functional improvement in ejection fraction of the left ventricle after four months, compared to those receiving a placebo treatment.
Tissue engineering of heart valves
A method for creating heart valves via tissue engineering was described by Kyoko Hayashida of the National Cardiovascular Center Research Institute (Osaka, Japan) that uses cells derived from adipose tissue to form valves in vivo. Hayashida has fabricated polyurethane molds with laser-drilled micropores having a diameter of 50 um to 100 um that induce adhesion and growth of stem cells from adipose tissue. The molds can be designed to grow both tubular structures for use as blood vessels (e.g., for dialysis access grafts or replacement of peripheral vessels) or more complicated structures with leaflets that can serve as heart valves. Both aortic and pulmonary valves have been fabricated, as well as trileaflet structures.
While the mechanical strength of the valve leaflets is somewhat less than that for biological valves derived from porcine tissue, they have sufficient strength to withstand the shear stresses encountered in vivo. The fabrication process involves implantation of the mold into the subcutaneous space in a layer of adipose tissue. In studies conducted in rats, the implants were placed in the animals' backs, and were sufficiently small (1 cm long and 1 cm in diameter) as to not interfere with normal activities of the animal. After one month, the mold is removed and the valve is extracted from the mold.
Paul DiMuzio of Thomas Jefferson University (Philadelphia) reported results of studies performed in patients over the age of 70 with cardiovascular disease to determine the levels of autologous stem cells present in adipose tissue compared to levels found in young, healthy patients. While the ability to harvest viable stem cells from adipose tissue has been demonstrated, the studies have mostly been performed in conjunction with cosmetic surgery procedures such as liposuction, whereas the most important applications of stem cell therapy, such as treatment of heart failure, are in older populations with disease.
One important application being targeted by DiMuzio is fabrication of a tissue-engineered, small-diameter vascular graft for use in dialysis access and vascular bypass. In a study of a group of 49 patients undergoing various cardiovascular procedures, DiMuzio's team collected stem cells from the patients' abdominal adipose tissue via standard liposuction techniques, and assessed viability of the cells by culturing in vitro for seven days. Not only were the cells found to be viable, but greater numbers of cells were found in patients with cardiovascular diseases than have been found in patients with other diseases.
The studies indicate that adipose tissue is a suitable source for stem cells for use in cardiovascular disease therapy. One issue uncovered by the study, however, is that diabetics have a significantly lower level of stem cells in adipose tissue, which could pose a barrier to using such treatments in diabetics with cardiovascular disease.
Fabricating devices for the heart
Tissue-engineering technology also is being applied to the fabrication of heart-assist devices for use in the treatment of heart failure. A research team led by Wolfram Zimmermann of University Medical Center Hamburg-Eppendorf in Germany is developing tissue-engineering techniques to fabricate a biological heart-assist device. The device is similar to the CorCap from Acorn Medical (St. Paul, Minnesota), but with active contractile capability. Zimmermann's group is not collaborating with Acorn in development of the device.
The CorCap device is a mesh wrap placed around the heart in a surgical procedure, and provides mechanical support to relieve muscle wall stress, helping to prevent and reverse HF progression by improving the heart's structure and function.
Acorn is pursuing approval via the FDA's dispute process following initial agency rejection.
The device being developed by Zimmermann would add engineered heart tissue to the basic concept of a mechanical support device to create a biological ventricular-assist device. So far, only animal studies have been performed. The fabrication process begins with heart cells derived from neonatal rats, which are seeded into a layer of Matrigel cell culture medium coating a mold. After culturing of the cells, a spheroidal tissue structure is obtained that can be slipped over an adult heart. The tissue has demonstrated the ability to contract and to respond to electrical pacing stimulus. Implants in rats have exhibited signs of vascularization, and the engineered tissue has been shown to remain intact in vivo when explanted at 14 days.
Juan Chachques of Georges Pompidou Hospital (Paris) presented recent results from the Myocardial Assistance by Grafting a New bioartificial Upgraded Myocardium (MAGNUM) clinical feasibility trial, which is evaluating a biomaterial comprised of bone marrow-derived stem cells seeded into a biodegradable Type 1 collagen matrix. The construct is implanted into an infarct region in the heart to provide structural support and confer shape changes that promote improved function, along with active contractile function provided by the implant itself.
The trial has enrolled 30 patients so far, with 15 receiving implants of the cell-seeded collagen matrix and 15 receiving injections of cells only. Both patient groups exhibited a similar degree of improvement in New York Heart Association function class compared to controls, dropping from 3.2 to 1.4 in the matrix group. Significant improvement in diastolic function was observed in both groups of patients at 12-month follow-up, but the improvement was greatest in the group receiving cell-seeded matrix implants. In addition, the infarct scar area was reduced by an average of 31% in the matrix implant group vs. 19% in the cell implant group.
Future developments will include implementation of features to enhance electrostimulation of the implant for improved contractile function, and subjecting the cell-seeded matrix to shear stresses prior to implant in order to confer more effective contractile and mechanical properties. A significant benefit of the matrix is that it enhances survival of the implanted cells, addressing a major drawback of alternative approaches that use only implantation of cells.
