BB&T Contributing Editor
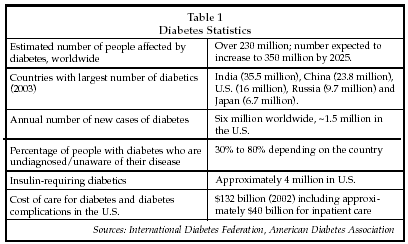
WASHINGTON – Diabetes is the second-largest category of chronic disease worldwide, and is one of the most rapidly growing categories of disease globally. As shown in Table 1, there are more than 230 million affected by diabetes worldwide including a large number outside the U.S., and the number is continuing to rise. A number of factors are responsible for the trends in diabetes incidence and prevalence, including the epidemic of obesity that is sweeping the U.S. and aging of the population in the developed regions of the world. Other factors that predispose individuals to develop diabetes include genetic determinants and rising living standards that lead to diet and exercise practices that make populations in developing countries more susceptible to developing the disease. Improved testing methods also make it easier to identify people with diabetes, although a significant number of diabetics remain undiagnosed.
While there is so far no permanent cure for diabetes, a wide range of new developments in the pharmaceutical and medical device sectors have enabled outcomes for diabetic patients to improve, simultaneously creating a large and growing market. As discussed here at the 66th annual scientific sessions of the American Diabetes Association (ADA; Alexandria, Virginia) in June, new technologies continue to be introduced that are expected to enable further improvements in outcome, by allowing patients to track their blood glucose levels more effectively, and by simplifying medication delivery. Advances in information systems and communications are enhancing the ability of physicians to monitor their diabetic patients and to assess the results of interventions. Important new developments in glucose monitoring technology also were described at the ADA meeting that will expand the market, with continuous glucose monitoring devices now a major emerging segment of the market.
Advances in disease management drive growth
A significant opportunity exists for improving the management of diabetes by identifying at-risk individuals at an earlier stage and initiating treatment. Treating diabetes is important not only because the disease itself can result in life-threatening metabolic imbalances, but also because of the serious complications associated with diabetes, which include cardiovascular disease as an element of the metabolic syndrome. As discussed by David Eddy, MD, of the Archimedes Project (Oakland, California) at an ADA press conference, if all individuals with metabolic syndrome in the U.S. could be detected and treated, the death rate for cardiovascular disease could be reduced by an estimated 63%. Associated healthcare costs could be reduced by an estimated $6.6 trillion.
Even if only those with insulin resistance were successfully treated, deaths could be reduced by 42%. Insulin resistance is very common in the developed world: about half of the population of the U.S. can be expected to develop insulin resistance at some point in their lives. Physicians discussing metabolic syndrome at the ADA conference believe that diabetes screening should be an integral part of the assessment of cardiovascular disease risk, since few individuals are now routinely assessed for the presence of diabetes. It is particularly important to identify insulin resistance at an early stage, since therapy and lifestyle modifications that can minimize the adverse effects of the condition are more effective the sooner they are implemented.
A new technology that shows promise for improving the detection of individuals with undiagnosed diabetes was described at the conference by Veralight Medical, a spin-off of InLight Solutions (Albuquerque, New Mexico). VeraLight is developing the Scout system, which employs fluorescence spectroscopy to noninvasively measure advanced glycation endproducts (AGEs) in the dermis of a subject’s forearm. The technique normalizes tissue optical properties between individuals by measurement of both excitation and emission reflectance spectra at multiple wavelengths, compensating for absorption differences due to melanin content (skin color) and blood supply plus scattering differences due to skin layer thickness and collagen organization.
The formation of AGEs in skin collagen and the accelerated accumulation of AGEs in subjects with diabetes are well established, and studies have found correlations between higher levels of specific skin AGEs and increased incidence of diabetes complications. For example, at the closeout of the Diabetes in Control and Complications Trial, the Skin Collagen Ancillary Study Group found skin AGEs, which act as an integrator of glycemic exposure, were positively associated with measures of diabetic retinopathy, nephropathy and neuropathy. VeraLight was formed in October 2004, and at that time estimated approximately three years to market launch for the Scout.
The VeraLight technology could improve the detection of diabetes significantly since existing diabetes screening methods (primarily the fasting plasma glucose test) require blood sampling and time commitment by the subject, whereas the noninvasive Scout system could allow screening to be performed in much the same way as noninvasive blood pressure measurements now used to screen for cardiovascular disease.
A wide variety of technology-based approaches are being applied to improve the management of diabetes. Information technology, for example, is playing an increasingly important role in diabetes management. One approach to applying information technology in diabetes management involves remote, real-time monitoring of glucose levels and insulin infusion via the cellular telephone network. Safe-com GmbH & Co. KG (Lichtenfels, Germany) exhibited its new GlucoTel system that consists of a glucose meter linked to a cellular phone by a wireless Bluetooth connection. Glucose readings taken by the meter are automatically transmitted to the cellphone, and then forwarded in encrypted form to a server where the data is stored in a web-accessible database. The patient also enters insulin dosage data into the phone which is transmitted to the database. The data can then be reviewed at a call center to track the patient’s history in achieving target goals for glycemic control. If adjustments in the patient’s diabetes management protocol are needed, the patient’s physician or disease management coordinator can call to prescribe the changes, such as an altered insulin dosage schedule.
The components of the GlucoTel system include the software used by the call center to acquire, process and display patient data; the glucose meter; and glucose test strips. Safe-com plans to provide the software at no charge, and will sell the meter for $30 or, in some cases such as large-volume purchases, provide it at no cost. The company’s goal is to sell test strips to patients who use its glucometer. The product was exhibited for the first time at the ADA conference, and will be launched nationwide in January 2007. Safe-com is searching for strategic partners and distributors to carry the product.
GlucoCOM, a division of Cardiocom (Chanhassen, Minnesota), exhibited the GlucoCom Telemonitoring System, a remote monitoring system that can be used to track the status of diabetic patients in the home. The system consists of the GlucoCom Blood Glucose Meter and test strips, the AutoLink Blood Glucose Telemonitoring device and a disease management program linked to a nurse call center that provides patient monitoring services.
The company also markets the Commander Multi-Biometric Telemonitoring Device, which allows a variety of cardiovascular and respiratory parameters to be monitored in addition to glucose. The system operates over phone lines, and provides another option for intensive tracking of glycemic control in diabetic patients in the home. The equipment is leased to disease management and other patient management companies starting at $16 to $18 per month for basic glucose monitoring services. Prices can range up to $135 per month for comprehensive monitoring of multiple metabolic, cardiovascular and respiratory parameters.
Another new technology that uses advanced IT to improve diabetes management was shown by GlucoTec (Greenville, South Carolina). The Glucommander is a dedicated portable tablet computer and software that is used to guide tight glycemic control (TGC) protocols in the acute-care hospital. A growing number of hospitals in the U.S. have implemented TGC protocols as a result of recent clinical studies that have shown in-hospital mortality reductions of 30% to 40% in critically ill patients, primarily in those with cardiovascular disease. TGC protocols employ intensive insulin therapy along with frequent bedside blood glucose measurements to maintain glucose levels in the normal range between 70 and 100 ug/dL, and in some cases in an even more narrow range.
GlucoTec has developed and patented key features of its software and systems, and recently obtained 510(k) market clearance for the Glucommander system. The Glucommander can be used to manage both intravenous and subcutaneous insulin infusion. The system will address a rapidly growing market opportunity for computerized guidance of insulin dosing protocols in the hospital, and is the only FDA-cleared system. The latter feature could make the system a preferred option vs. using non-cleared computer systems developed by some hospitals for TGC, or vs. manual computation to derive dose levels. Vendors say about 20% of hospitals in the U.S. have adopted TGC protocols, but given the benefits in patient outcome and potential cost savings for insurers, it is likely that such protocols will eventually be used in all hospitals that manage acutely ill patients.
Rapid growth for insulin delivery
Insulin delivery products comprise a growing segment of the diabetes device market. Devices such as insulin pumps and pens are being used by an increasing number of insulin-requiring diabetics. Leading suppliers of pumps include Medtronic Minimed (Northridge, California), Roche/Disetronic (Indianapolis); Animas (West Chester, Pennsylvania), now a unit of LifeScan/J&J (Milpitas, California); Smiths Medical/Deltec (St. Paul, Minnesota), and SOOIL Development Co. Ltd. (Seoul, South Korea).
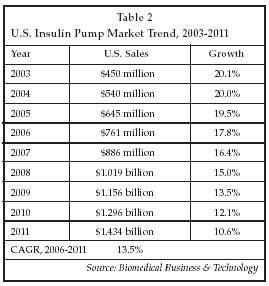
As shown in Table 2, the market for insulin pumps and disposable infusion sets is estimated at $645 million in the U.S. for 2005, growing at about 20%. Worldwide, the 2005 market is estimated at some $930 million. About 70% of the market is attributable to pump sales and the remaining 30% to sales of disposable infusion sets and related supplies. Pumps are typically priced at about $5,200 to $5,400, and sets cost $12.50 to $14 each.
A new development in the insulin delivery device market is the introduction of set insertion devices that can be used to facilitate the process of replacing the disposable infusion set, a procedure that must be performed every three days. Smiths Medical has introduced the CLEO 90 insertion device for use with its Cosmo insulin pump. The $13-to-$14 device uses a shielded needle in a self-contained cartridge that also contains the cannula and the Flex-Attach 360 site connector with adhesive backing. The user removes a cap and actuates the insertion device to place a cannula and the connector, and then attaches the set tubing to the connector and pump. The tubing can be connected in eight different orientations. So far, about 2,500 patients who use the Cosmo pump have adopted the CLEO 90. Animas also has introduced a set insertion device, priced at $13, for use with its IR 1000 and IR 1250 insulin pumps.
The insulin delivery market is now poised for major changes due to competitive factors as well as the entry of new technologies. Competition in the insulin pump sector will likely intensify as a result of strategic moves by major players in the industry. Medtronic, which has so far dominated the market particularly in the U.S., will face a growing challenge from LifeScan/J&J as the latter company ramps up its marketing efforts behind its newly acquired Animas pump line, which presently has about 12% of the U.S. market. LifeScan may have a particularly attractive opportunity to expand sales of the Animas line outside the U.S., a segment that at present accounts for less than 10% of Animas’ sales.
Other challenges to Medtronic in the U.S. are expected from overseas, as Roche introduces its AccuChek Spirit pump, which will have considerably enhanced features compared to the company’s D-Tron pump introduced into the U.S. initially by Disetronic prior to its acquisition by Roche. Roche already has launched the Spirit outside the U.S., and now has 15,000 users worldwide. Yet another challenge will come from SOOIL, which plans to introduce the new DANADiabecare IIS pump in the U.S. pending FDA clearance. SOOIL already sells the DANA Diabecare II pump in the U.S., priced at $4,000 (vs. $5,400 for the Medtronic Paradigm), along with sets priced 25% to 35% below those from other pump manufacturers. The new pumps from SOOIL will add features such as a carbohydrate counter, dual-wave bolus and a built-in glucometer, as well as an autodose feature. SOOIL expects to achieve significant gains in the U.S. market once its new pumps become available.
Another challenge to existing leaders in the insulin pump market will come from new types of insulin delivery technology. The most important development is the introduction of inhalable insulin, in the form of a new product launched by Pfizer (New York) at the ADA conference. The product, Exubera, was jointly developed by Pfizer and Nektar (San Carlos, California), and has the potential to revolutionize the insulin delivery market by eliminating the need for injections or insulin pumps. The product consists of a manually actuated canister and individual blister packs containing insulin in powder form. To receive a dose of insulin, the user pulls up a plastic chamber and inserts an insulin pack, pushes a handle to pressurize the system, and then presses a button to release insulin into the chamber. The user immediately inhales the contents of the chamber, receiving a 3- or 9-unit insulin dose.
Exubera is intended for use by both Type 1 and Type 2 diabetics, and a pilot study using the device has shown that patients can achieve a significant drop in glycated hemoglobin (1.9%) with 12 weeks of use..
Additional inhalable insulin systems are being developed by Eli Lilly (Indianapolis) in partnership with Alkermes (Cambridge, Massachusetts) and by Novo Nordisk (Bagsvaerd, Denmark) in partnership with Aradigm (Hayward, California). Inhalable insulin may prove a viable alternative to insulin pumps for some patients, but the primary users are expected to be those patients who currently are taking oral diabetes medications as well as those who are taking injectable insulin plus oral agents. Consequently, the total insulin delivery market is likely to expand as a result of the introduction of inhalable insulin. The primary limitation of inhaled insulin technology is the contraindication for use in patients with respiratory conditions.
Another new insulin delivery technology was introduced late last year in the U.S. by Insulet (Bedford, Massachusetts). The company’s OmniPod employs a next-generation design that eliminates the need for tubing and connectors used with existing insulin pumps. It consists of a pod that is attached to the skin with a self-adhesive backing that contains an integrated cannula that is automatically inserted when the pod is applied to the skin. The pod also contains a miniaturized pump mechanism that employs shape memory alloy technology (a nitinol wire) to drive a piston and deliver insulin. The pod measures 1.6 x 2.4 x 0.7 inches, and weighs 1.2 ounces with a full insulin reservoir. A small hand-held controller, the PDM, guides the user through the process of applying the pod, and controls all functions of the pump via a wireless interconnection. The PDM also includes an integrated FreeStyle glucose meter supplied by Abbott, eliminating the need to carry a separate meter. Like existing insulin infusion sets, the pod must be replaced every three days. Overall cost for the OmniPod system is similar to that for other insulin pumps that use tubing sets at $325 per month, but the upfront cost is considerably less at $800, since the PDM is the only equipment required. The monthly cost is equal to the established reimbursement rate set by Medicare for insulin pumps and sets in the U.S.
At present, Insulet is selling the OmniPod only in the U.S., with sales currently focused on the East Coast. Insulet was founded in 2000, and now has 130 employees. The company is venture-funded, and has raised $120 million so far.
Another disposable insulin pump is under development by Debiotech (Lausanne, Switzerland) that is fabricated using MEMS technology. The Debiotech Insulin Nanopump incorporates a painless microneedle for insulin delivery, and can carry an amount of insulin adequate for seven days of therapy.
Continuous glucose monitoring expands
Continuous glucose monitoring was introduced over five years ago with the launch of the Medtronic Minimed Continuous Glucose Monitoring System (CGMS). The CGMS launch has since been followed by the introduction of a new continuous monitor from Dexcom (San Diego), as well as a monitor employing microdialysis technology, the GlucoDay from Menarini (Florence, Italy). Other continuous monitoring systems are being developed by leaders in glucose self-testing, including Abbott and Roche, as well as by new entrants in the glucose testing market as shown in Table 3.

Numerous clinical studies have shown that continuous glucose monitors can detect more hypo- and hyperglycemic events than fingerstick glucose testing. Diabetics who have used continuous monitors such as the new Dexcom STS have achieved reduced duration of hypo and hyperglycemic events, and have succeeded in lowering their hemoglobin A1c levels below those they were able to achieve using fingerstick monitoring. Existing continuous monitors measure subcutaneous glucose levels using a sensor inserted under the skin, and report updated readings about every five minutes.
New systems under development, such as the Animas continuous glucose monitor, will track glucose levels in blood, potentially eliminating the delay between subcutaneous and blood or plasma glucose levels. Existing continuous monitors also require that the user perform a fingerstick glucose test every 12 hours for calibration. Medtronic has just introduced a next-generation continuous monitor as part of its Paradigm REAL-Time system, which combines an insulin pump with a continuous glucose monitor. The subcutaneous sensor is connected to a miniaturized transmitter that is taped to the skin, and glucose results are transmitted to the pump for display. The transmitter is priced at $999, and glucose sensors cost $10 per day, or $30 for a three-day interval.
Abbott’s Navigator, which is under review by the FDA and expected to be available for sale in the U.S. by year-end, will allow monitoring for up to five days with a single sensor, vs. only three days for the Medtronic and GlucoDay systems. The Navigator updates the glucose reading every minute, in principle allowing more precise adjustment of insulin doses. Alarms to detect certain thresholds can be set, and only one fingerstick calibration will be needed per day of use.
Two new studies of the Navigator were presented at the ADA conference. One was conducted by the DirecNet study group and involved 30 children ages 4 to 17. Accuracy and precision were maintained throughout the five-day period of use, and did not vary between home and inpatient use. In the second study, which involved a comprehensive evaluation of precision and accuracy, the mean absolute difference between the Navigator readings and reference fingerstick blood glucose values was 14.4%, and 96.8% of the values fell in zones A or B of the Clarke Error Grid.
International Biomedical, which acquired VIA Medical (San Diego), a supplier of continuous blood analyte monitoring systems, exhibited the VIA Blood Glucose Monitor, which is being re-introduced to the market because of the growing interest in continuous monitoring in the hospital to obtain improved glucose data for TGC protocols. The VIA monitor is only used in the hospital critical care setting, since it requires an indwelling catheter to be placed and is not intended to be carried by the patient.
The Dexcom STS System offers diabetics the capability to use continuous monitoring independent of a physician or nurse, since the subcutaneous sensor can be inserted and removed by the patient. A starter kit including the receiver, transmitter, two sensors, and battery charger is priced at $800, and sensors with a three-day life are priced at $35 each. The system was cleared for marketing in March by the FDA, and the product is now being sold nationwide in the U.S.
Another new device for monitoring hypoglycemia was described at the ADA conference by AiMedica (Sydney, Australia). The company’s HypoMon monitor does not measure glucose levels, but instead is a noninvasive monitor of physiological parameters including skin impedance and ECG parameters that can be used as indicators of a hypoglycemic event. The system has been under development for over two years, and has shown promise for alerting diabetics of hypoglycemic events that are not recognized and therefore create a potentially dangerous clinical situation. A study presented at the conference showed that the HypoMon can detect hypoglycemia with a sensitivity of 78% and specificity of 95%. The monitor is primarily being evaluated for use in nocturnal monitoring, when diabetics are least able to detect the onset of hypoglycemia.
In spite of the introduction of continuous glucose monitors, which offer advantages over fingerstick testing by allowing closer tracking of glycemic status, the market for conventional glucose meters and test strips is expected to continue to remain strong over the next few years. Continuous monitors, because of their cost, will not be appropriate for all diabetics, particularly for the large number of Type 2 diabetics who test only once or twice per day. In addition, existing continuous monitors as well as development-stage continuous monitors expected to enter the market in the near future continue to require diabetics to perform fingerstick testing. As shown in Table 4 below, while growth in the market for glucose meters and test strips is expected to moderate from the 15% to 20% annual increases of a few years ago, market growth is nevertheless expected to remain well above that for the medical device market overall.

Among the new products exhibited at the ADA conference were the OneTouch Ultra2, a LifeScan meter launched in March that allows storage of before and after-meal results and also computes average glucose levels. Recent studies have shown that use of meters such as the LifeScan UltraSmart that provide data to users in a form that facilitates behavioral changes can have quantifiable effects on diabetes outcome. In the case of the UltraSmart, a study cited by LifeScan has demonstrated a reduction of hemoglobin A1c from 9.1% to 8.7% through use of the meter.
The glucose meter market also is becoming increasingly competitive, in part as a result of the entry of numerous small suppliers from Asia. For example, new glucose test systems were exhibited by Invictus Scientific (San Diego) that are manufactured in Taiwan by Bionime (Taichung County, Taiwan). Asian suppliers also have entered the European market and other international markets in large numbers recently, a trend that will exert downward pressure on prices and likely limit market growth in the long term.
A number of companies also are continuing to develop noninvasive glucose monitoring technologies, which could potentially replace both continuous glucose monitors using subcutaneous or implantable sensors as well as conventional whole blood glucose meters and test strips. So far, however, the goal of developing a noninvasive glucose monitor having the accuracy required for patient use has proven elusive.
Glucon (Boulder, Colorado), one of the companies involved in the development of noninvasive glucose monitors, reported the results of a new clinical study at the ADA conference using its Aprise system, which uses photoacoustic technology to isolate optical signals from veins in order to improve signal-to-background ratio. Glucon believes its Aprise monitor is the most advanced noninvasive glucose monitor in the clinical investigation process. The study was conducted in 62 subjects and involved 979 pairs of sensor and reference readings. Analysis of all results obtained in the study showed that 94.1% of the points were in zones A and B of the Clarke Error Grid, with a mean absolute relative difference of 19.9%.
Reports on progress in the development of other noninvasive glucose monitoring technologies were given at the ADA conference by Lein Applied Diagnostics (Berks, UK) and OrSense (Rehovot, Israel). Lein Applied Diagnostics is developing the OneLook, a hand-held portable optical reader that analyzes reflected spectral data from the aqueous humor of the eye. The device uses confocal optics and can perform a scan in 0.1 seconds. A four-hour comparison study of 17 subjects including 10 diabetics and seven controls found a 15% mean absolute error between the OneLook readings and fingerstick glucose values, and a correlation coefficient of 0.93.
OrSense presented results of a study involving 12 subjects in three settings including the clinic, a home-like setting, and an intensive care setting, using its NBM-100 occlusion spectroscopy analyzer. Data was collected in a total of 52 sessions, and 87% of the data points were used in the analysis. The study found a mean absolute difference of 12.9% compared to fingerstick readings, a correlation coefficient of 0.84, with 95.3% of the results falling in zones A and B of the Clarke Error Grid.
