BBI Contributing Editor
SALT LAKE CITY, Utah The clinical laboratory market, which includes a broad spectrum of laboratories in hospitals, physicians' offices, clinics and a widening array of other sites such as pharmacies and nursing homes, is experiencing increasing demand for testing services, while simultaneously facing a growing shortage of resources to cope with the rising workload. The dynamics of the industry are creating a growing opportunity for product suppliers who can provide tools to enhance the productivity not only of laboratories, but also of the extended infrastructure involved in screening, diagnosis, and monitoring of patients via in vitro diagnostic techniques.
The annual meeting of the Clinical Laboratory Management Association (CLMA; Wayne, Pennsylvania), held here in the latter part of June in partnership with the American Society for Clinical Pathology (Chicago, Illinois), provided a forum for discussion of the changes occurring in the laboratory industry in response to market pressures, as well as a venue for suppliers to exhibit solutions for labs seeking to enhance productivity and expand the range of services they offer.
As discussed by healthcare futurist Jeff Goldsmith, PhD, the clinical laboratory may be on the threshold of a new era in which diagnostic testing becomes an increasingly critical component of the healthcare system, due to increasing use of technologies such as genetic testing, pharmacogenetic testing, and tests for monitoring patients with chronic diseases such as diabetes, heart failure and cancer. Diagnostics is expected to become an integral part of treatment in the future, in part to help reduce adverse events that now plague health are providers. For example, Goldsmith cited a recent study that found that the incidence of sepsis in U.S. hospitals approximately tripled between 1979 and 1997, indicating serious quality issues in healthcare institutions. More effective use of diagnostic testing, both in the laboratory as well as at the point of care, can potentially improve patient management, reducing medical errors, as well as the use of inappropriate therapy, while also helping to improve the efficiency of healthcare.
Molecular diagnostics is playing an increasingly important role in the lab, both as a fundamental technology required for genetic and pharmacogenetic testing, as well as in areas such as cancer screening and diagnosis, infectious disease testing and detection of coagulation disorders.
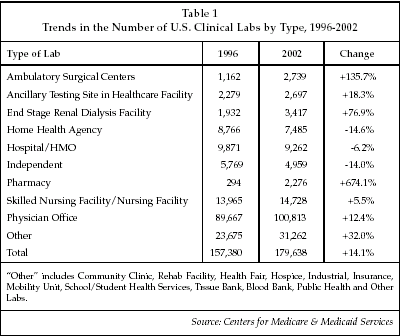
The number of laboratory facilities has continued to increase over the past few years, according to data from the Centers for Medicare & Medicaid Services (CMS; Baltimore, Maryland), particularly for labs in certain non-hospital settings such as pharmacies, renal dialysis facilities and ambulatory surgery centers. As shown in Table 1, the number of pharmacy labs increased seven-fold between 1996 and 2002, based on totals provided in January of the following year by CMS. Pharmacies are now offering a wide range of tests, usually those that are waived under the CLIA '88 regulations, that can identify individuals who may benefit from drug therapy, such as lipid-lowering therapy, or that can help patients who are taking certain types of drugs in monitoring the effects of treatment. Another area attracting strong interest is bedside systems that can be used in the hospital setting to guide the collection of blood samples to help prevent misidentification of specimens, and provide more efficient routing of samples to the lab as well as capture information on test activity to avoid missed charges. A considerable level of attention is now directed at improving the efficiency and safety of blood transfusions in the hospital, via the implementation of automated systems for sample collection as well as for pre-transfusion testing in the lab, and one leading supplier of blood bank testing products launched a new automated system at the CLMA meeting.
New products also were introduced or previewed for automating cellular/microscopic analysis procedures such as the Pap test, immunofluorescence slide test and body fluid analysis. A shortage of personnel qualified to perform microscopy tests is forcing labs to automate such procedures, providing a growth opportunity for suppliers, but offering benefits for the lab in terms of improved accuracy and, in many cases, cost savings.
LIS, bedside IT products make inroads
About 40 companies now market laboratory information systems in the U.S., including Cerner (Kansas City, Missouri), Misys Healthcare Systems (Tucson, Arizona), Park City Solutions (Midway, Utah), Opus Healthcare Solutions (Austin, Texas), SCC/Soft Computer (Clearwater, Florida), McKesson Information Solutions (Alpharetta, Georgia), 4Medica (Culver City, California), Orchard Software (Carmel, Indiana), Wyndgate Technologies (El Dorado Hills, California), Impac Medical Systems (Mountain View, California) and Sysware (Farmington Hills, Michigan). Another supplier, Triple G Systems (Markham, Ontario), recently agreed to be acquired by GE Medical Systems (Waukesha, Wisconsin), for $55 million. The LIS market now totals about $500 million worldwide, according to suppliers, about 1% of the total healthcare IT market. Leading suppliers include Cerner, Misys, SCC, Triple G and McKesson.
Although the overall healthcare IT market is continuing to grow, with analysts predicting expenditures by U.S. healthcare providers to increase from $21 billion in 2001 to $32 billion by 2005, the LIS market is not growing rapidly. Suppliers offering only LIS products are dwindling, with the leading companies in the LIS segment also offering information systems products for radiology, pharmacy, and other specialties.
A major trend in the LIS/clinical information systems market is the addition of real-time automation of the specimen collection process at the bedside. Lattice (Wheaton, Illinois) was the first supplier to offer bedside systems using barcode technology to automate clinical processes such as patient identification and blood sample tracking. The Lattice MediCopia system was first introduced in June 1996. It includes a $5,000 hand-held unit and portable printer. Recently, many LIS and clinical information suppliers have begun to implement similar technologies. McKesson, for example, introduced a new wireless version of its Horizon MobileCare Phlebotomy system at the CLMA exhibition that features a hand-held computer from Symbol Technologies (Holtsville, New York) which can be used along with a portable printer to track and match the patient, specimen and test results using bar code technology. The system can be used at the bedside to scan the patient's barcode wristband, as well as a barcode badge identifying the phlebotomist, and to print a specimen label at the bedside, which is applied to the tube drawn from the patient. The ability to positively identify the patient and the specimen addresses a significant source of errors associated with lab testing in the hospital.
Recent studies have shown that 68% of lab errors are related to pre-analytical procedures, and that about half of those errors are the result of misidentification of the patient or mislabeling of the specimen. Consequently, systems such as MobilCare Phlebotomy can potentially reduce lab errors by more than one-third. The cost for MobilCare Phlebotomy is between $1,500 and $2,000 per bedside device, plus an installation cost of $15,000 to $20,000 depending on the number of devices deployed. An add-on now in development will automate blood transfusion operations at the bedside. The MobilCare system also includes modules for tracking medication delivery, for downloading of lab results to a physician's hand-held computer, and for physician messaging, including automatic alerting to a cellphone or handheld computer when a patient's results are out of range. Long-term, McKesson is evaluating the addition of genomic and proteomic data to its LIS capabilities and is just starting to assess the data structures that will be needed to handle genomic data for use by physicians at the bedside.
Another trend in the LIS industry is the implementation of web-based order entry and result reporting systems. Misys (formerly Sunquest) has introduced Compass, offering a web interface to its clinical information system, including LIS capabilities. Misys Insight, developed in collaboration with the University of Utah Health Sciences Center (Salt Lake City, Utah), not only provides point-of-care capabilities, but also offers computerized decision support resources that can, for example, alert hospital staff when a patient is admitted who requires special handling based on past history. Web-based interfaces are becoming increasingly important as a tool to allow easy access to the LIS from remote sites, without the need to install specialized, dedicated terminals and train a large and constantly changing user base. Suppliers such as Medicity (Salt Lake City, Utah) now offer a completely web browser-based LIS, and in addition are developing Windows-based handheld computer interfaces that provide point-of-care capabilities. Windows-based platforms are becoming increasingly popular, because of the ease of training new users and because of the ability to employ standardized hardware that reduces acquisition and support costs.
Sysware, a rapidly growing supplier with about 50 installed clients, including the Alabama public health system with 125 clinics and five core labs, has developed an all-Windows LIS platform called PowerLAB, which runs on standard hardware from Dell Computer (Austin, Texas) and allows upgrades such as interfacing of additional instruments at considerably lower cost than most other LIS vendors. Because of the use of standardized hardware and readily scalable software technology, Sysware can typically complete a new LIS installation in six weeks, at a cost of $250,000 to $2 million, with a low ongoing cost of ownership. The system offers real-time interfaces to point-of-care instruments, as well as web-based order entry and reporting. Most Sysware installations have been in non-hospital labs so far, including clients such as LipoScience (Raleigh, North Carolina), a specialty reference lab. Now, however, the company is beginning to emphasize placements in the hospital lab segment, and has just introduced an anatomic pathology module, APara, that offers integrated image capture and voice dictation, paperless workflow and the ability for simple customization of the user interface by the customer. Another module now under development, PowerBank, is designed to automate data management in the hospital blood bank by providing electronic cross matching, blood product inventory management, and maintenance of quality testing information.
Growth in automated transfusion systems
Blood bank automation is, in fact, emerging as a growth segment in the laboratory products industry. Blood banks have been one of the last segments of the clinical lab to adopt automation, in part because of the relatively small size of the market segment (about 2% of the total in vitro diagnostic products market) and low test volume as compared to areas such as clinical chemistry, immunoassay and hematology. The first company to begin offering automation for blood bank testing was Immucor (Norcross, Georgia), which launched the ABS2000 instrument in mid-1998 in the U.S. Although product problems including a recall in July 2000 slowed adoption over the first few years, a number of labs are now using the Immucor instrument and achieving good results in addition to significant cost savings. In large part, the increasing demand for automation in the blood bank is the result of the shortage of technologists and technicians throughout the clinical laboratory industry, coupled with increasing test demand of up to 35% over the past four years in some cases. In addition, staff expertise in specialty areas such as blood banking may be diminishing in some labs, adding to the difficulty of simply increasing the number of technologists to meet increased demand.
Ortho-Clinical Diagnostics (Raritan, New Jersey) introduced a new system for blood bank automation, the ProVue, at the CLMA meeting. The ProVue is manufactured by Micro Typing Systems (Pompano Beach, Florida) and distributed by Ortho along with the Ortho line of blood bank reagents. At present, Immucor and Ortho are the only two suppliers of a complete line of blood bank reagents in the U.S. The ProVue, which is priced at $120,000 (similar to the ABS2000), uses test cards that use the patented GelTest technology developed by Ortho. There are currently more than 1,800 users of the GelTest technology, which is based on column agglutination, in North America. The user only needs to add cells to the test card, and the ProVue test procedure requires only three steps vs. 21 when performing manual testing. In addition, the automated analysis of the agglutination reaction eliminates the subjectivity associated with manual testing. Up to 48 sample tubes can be loaded onto the instrument, and separate cameras are used to identify the sample, the test card and reagents, and to perform image analysis of the aggregation reaction. The ProVue offers random access testing including stat interrupt, automatic dilution and on-board centrifugation. Immucor has developed a second-generation analyzer, the Galileo, that provides full automation of blood bank testing, and that is now being marketed in Europe.
Automation in the blood bank market appears to be a win-win situation for both the laboratory and for manufacturers. Data presented at a CLMA breakout session for a lab using the Immucor ABS2000 demonstrates a savings of 18% in per-test cost ($3.42 vs. $4.15) for automated versus manual testing, resulting in total annual savings of more than $10,000 for the lab. Cost factors, labor shortages, and the ability to obtain more objective results with automated testing are expected to drive strong growth for product suppliers in the blood bank market. According to Immucor, the existing worldwide market for blood bank reagents is about $380 million, and labs spend an additional $1 billion in labor costs to perform blood bank tests. By switching to automation for the 37% of billable transfusion-related tests that can be run on systems such as the ABS2000 and ProVue, the available market for traditional and automated blood bank reagents and systems could expand to $575 million, while reducing labor costs by $500 million, thus reducing the overall cost of blood bank testing for the hospital.
Automation, test demand expanding
Molecular diagnostics is another segment of the lab where demand for automation as well as demand for testing is increasing rapidly. Although true automation for molecular testing has been slow to develop, suppliers such as Roche Diagnostics (Indianapolis, Indiana), BD (Franklin Lakes, New Jersey), Abbott Diagnostics (Abbott Park, Illinois) and Gen-Probe (San Diego, California) have collectively placed more than 10,000 analyzers offering various levels of semi-automated testing for nucleic acid-based assays. Recently, the market share structure in molecular diagnostics began shifting as a result of Abbott's decision to discontinue its LCx system and reagents due to manufacturing issues (effective at the end of June), creating an opening in the market for other suppliers. BD, for example, which has placed more than 1,000 of its ProbeTec molecular diagnostics systems worldwide, including over 700 in the U.S., now expects revenues for that product line to increase to $50 million this year, partly as a result of the opening created by the exit of Abbott.
As discussed by Silvia Spitzer, PhD, of State University of New York at Stony Brook (Stony Brook, New York) at a CLMA breakout session, molecular diagnostics is now expanding from its established base in infectious disease testing to include tests such as cystic fibrosis screens, tests for Trisomy 21 (Down's syndrome), and other genetic disorders, as well as oncology tests for targets such as Her/2-neu, BRCA1/2, gene translocations in chronic myelogenous leukemia and oncogenes such as p53 and retinoblastoma (rb). In addition, there is significant and growing demand for molecular tests for hemostasis disorders, including assays for Factor V Leiden and the prothrombin 20210 marker. The highest-volume tests, according to Spitzer, include infectious disease assays for chlamydia trachomatis, gonococcus, HIV including viral load, CMV, cystic fibrosis, Factor V Leiden and PT 20210. Cystic fibrosis testing has provided a significant stimulus for the molecular diagnostics market as a result of recommendations from the American College of Obstetricians and Gynecologists (Washington) for genetic screening of newborns. As shown in Table 2 on page 220, five suppliers now offer genetic tests for cystic fibrosis. None are offered as 510(k)-cleared kits, but rather as analyte-specific reagents (ASRs), which require the lab performing the test to perform validation before offering the test to physicians. Cystic fibrosis testing was initially offered primarily to those with a family history of the disease or to couples having specific ethnic backgrounds. However, since 80% of babies with CF are born to parents without a prior history of the disease, testing has expanded to the general population, driving a major increase in test volume.

Real-time PCR molecular assays represent another growth area, according to Spitzer. The technology provides faster turnaround times (one to two hours for reaction and analysis) since detection is performed while the amplification reaction is proceeding. Equipment used to perform real-time PCR tests includes the ABI 7700 from Celera Diagnostics (Alameda, California)/Applied Biosystems (Foster City, California), the SmartCycler from Cepheid (Sunnyvale, California), the LightCycler from Roche and the Q-PCR system from Stratagene (La Jolla, California). Most labs use either the LightCycler or the SmartCycler. Real-time PCR tests can be used, for example, to detect the chromosome 9/22 translocation in chronic myelogenous leukemia patients, a test that is becoming more important with the introduction of the drug Gleevec, a compound developed by Novartis (Basel, Switzerland). Detection of the translocation, which can be performed with a sensitivity of one in 100,000, is correlated with a 75% occurrence of relapse.
Yet another major and growing application for molecular testing is detection of the Factor V Leiden mutation in trauma and fracture patients, as well as in pregnant women, patients about to undergo surgery, and patients undergoing hormone replacement therapy. The Factor V Leiden mutation is a major cause of deep vein thrombosis in such patients, so testing can allow preventative measures to be taken in those who have the defect. At present, according to Spitzer, about 40% of labs that perform Factor V Leiden testing use PCR assays developed in-house (home brew tests), 25% use the Invader assay, 16% use the Roche LightCycler, and the remainder use other techniques. However, the market is moving toward greater use of the Invader and LightCycler tests because they are less labor-intensive than most home-brew assays.
In the future, chip-based molecular diagnostic systems are expected to become important in the clinical lab. As an indicator of that trend, Roche Diagnostics recently announced the introduction of the AmpliChip CYP450, which allows detection of two genetic mutations (CYP2D6 and CYP2C19) involved in the metabolism of drugs used in the treatment of many common diseases. The AmpliChip uses gene chip technology developed by Affymetrix (Santa Clara, California), and will be marketed as an ASR. Roche expects sales of the product to exceed $100 million by 2008.
Molecular testing also has become an important component of cervical cancer screening, since studies have shown that essentially all cases of cervical cancer can be traced to infection with human papilloma virus (HPV), and HPV is detectable with high sensitivity using molecular techniques. Digene (Gaithersburg, Maryland) is the leading supplier of HPV test kits for clinical use, reporting product sales of $45.8 million in 2002, up 40% vs. 2001. Sales increased 46.7% in the prior year. Sales of HPV kits accounted for 74% of Digene's revenues in fiscal 2002. Digene has established a partnership with Cytyc (Boxborough, Massachusetts) to jointly market its Hybrid Capture II HPV test along with Cytyc's ThinPrep Pap test.
Developments in automated cellular analysis
To further improve the efficiency of the PAP test, Cytyc also just launched the ThinPrep Imaging System, a new automated cellular imaging product that reduces the number of fields of view that must be reviewed on each Pap slide by a cytotechnologist from 120 to 130 down to 22. A study demonstrated a 71.6% reduction in fields of view reviewed, and an increase in the number of slides analyzed per day from 71 to 154, a 114.9% improvement. The ThinPrep Imaging System has received FDA and CLIA clearance for a daily workload limit of 200 slides, vs. the current limit of 100 per day for manual review. In an even more favorable development for the lab, CMS has established a new reimbursement code for computer-based Pap screening with a rate of $37, vs. the rate for manual screening of $28. Since the per-test cost using the ThinPrep Imager and test kit is $9, and labor cost with the automated imager is reduced by half (from $9 to $4.50), Cytyc estimates that the average lab will increase its profit per slide from $8 to $12.50, equivalent to an increase in profit margin from 28.6% to 41.9%.
Performance also is improved with the new automated system. A pilot study of the analysis of ASCUS slides with the ThinPrep Imager demonstrated a 6.4% absolute increase in sensitivity (from 75.6% to 82%), with no drop in specificity, and equivalent or better performance for the automated imager in the analysis of other types of Pap slides. The ThinPrep Imager in conjunction with the ThinPrep slides was able to detect 100% of 33 specimens know to have cancer cells present, reducing the false negative rate by 40% as compared to manual screening. With the compelling combination of improved screening performance, higher profitability, and enhanced cytotechnologist efficiency, there is a strong incentive for labs now using manual screening to adopt automation. Furthermore, as shown in Table 3, the number of Pap tests performed remains at well over 100 million worldwide while the number of qualified cytotechnologists has declined as indicated by the trend in the number of training programs in the U.S. At present, 35% of Pap testing labs have at least one vacancy, and the recruiting time to fill a cytotechnologist position has increased from eight to 20 months, coupled with an 11% average annual increase in salaries. As a result, many labs are likely to be forced to adopt automated Pap analysis in order to meet demand.

TriPath Imaging (Burlington, North Carolina) also markets an automated Pap imaging system, along with the SurePath liquid-based Pap test. TriPath's FocalPoint automated imager reduces cytotechnologist workload by eliminating the need to review 25% of the slides that are found to be normal by automated imaging. The system also provides a ranking of the remaining 75% to assist the cytotechnologist in the manual review. A new product now in development for the U.S. market and available outside the U.S., the FocalPoint GS receives electronic data from the primary computerized screen and identifies the 10 fields most likely to have an abnormality on each slide for review by the cytotech. The combination of automated primary screening coupled with a greater than 10-fold reduction in the number of fields reviewed on the remaining 75% of slides is expected to result in a three-fold reduction in overall cytotechnologist labor. Data for the version of the product now marketed outside the U.S. demonstrates an improvement in sensitivity. About 50 systems have been installed so far in Europe, Asia and Australia.
Cytyc's report that it had shipped its first ThinPrep Imager system was accompanied by a lawsuit filed by that company against TriPath, a move intended to counter a threatened suit against Cytyc by Tripath. TriPath is claiming patent infringement and a number of other unfair practices against Cytyc, while Cytyc is claiming the TriPath patents are invalid. While the basic functions of the Cytyc and TriPath systems have similarities, there also are some notable differences. The ThinPrep Imager does not rank fields of view based on their degree of abnormality, but instead scans the slide in a simple geographic fashion to minimize the movement required between fields to be viewed. Abnormal cells are physically marked on the Cytyc slide, whereas the TriPath system relocates abnormal areas for review by electronic means.
Other competitors in the automated cell imaging market include Applied Imaging (Santa Clara, California), Cellavision (Lund, Sweden), Chromavision Medical Systems (San Juan Capistrano, California) and Hyperion (Miami, Florida). Those suppliers have not chosen to enter the automated Pap imaging market, instead focusing on applications in cancer diagnosis (Chromavision, Applied Imaging), genetic analysis (Applied Imaging), white cell differential analysis (Cellavision) and automated analysis of immunofluorescence assay slides used to test for anti-nuclear antibodies (Hyperion). While sales for those applications are considerably smaller than in the automated Pap analysis segment, the market is exhibiting rapid growth. Most of the suppliers in the cell imaging market are developing new applications in areas such as breast cancer screening and diagnosis, colorectal cancer screening and ovarian cancer screening, indicating continued expansion of the market.
