BBI Contributing Editor
NEW ORLEANS, Louisiana — The clinical laboratory plays a key role in the U.S. healthcare system, with laboratory data used in making 60% to 70% of all clinical decisions in the hospital setting. However, like most other segments of the healthcare industry, the clinical lab has been under intense pressure to contain costs over the past few years, leading to considerable consolidation of laboratory testing.
Recently, some positive trends have begun to appear. The resurgence of healthcare cost increases is now a well-established trend in the U.S. economy, with insurance premiums rising at rates of 20% to 50% per year and national expenditures for healthcare increasing at double-digit rates. The additional flow ofspending into the healthcare market has begun to create some positive trends for laboratories. However, some segments of the healthcare provider industry have not, at least so far, benefited from the recent growth in spending. For example, physicians are slated to absorb a 5.6% cut in pay for services provided to Medicare patients this year, and could be hit with reductions totaling 18% over the next three years, although those changes are likely to be revised.
As discussed here by Dennis Weissman of Washington G-2 Reports during the late-June annual meeting of the Clinical Laboratory Management Association (CLMA, Wayne, Pennsylvania), the reimbursement and regulatory picture for the clinical laboratory services industry is beginning to show some signs of a turnaround. For manufacturers of clinical diagnostic products, there are also some positive trends in the market, particularly for suppliers of lab automation products because of rising test volume coupled with a severe and worsening shortage of lab personnel.
One area that is attracting significant interest is lab informatics, a technology that cannot only help to improve efficiency within the laboratory industry and in health care in general, but that also can help address issues with the quality of healthcare. Advances in information systems are already helping labs reduce the cost of operations by streamlining charge capture and billing processes, and providing a tool to improve quality control in the central lab as well as at the point of care. Informatics technologies, particularly web-based approaches, are allowing the laboratory to extend its reach to an increasing range of sites, changing the structure of diagnostic testing. Informatics for improving quality control is proving particularly valuable in the alternate site setting, helping to address significant issues with test quality that have attracted growing attention recently as a result of government investigations.
Another area that is benefiting from advances in informatics and automation is cellular analysis. New imaging systems and specimen processing devices are now finding more widespread acceptance as hospital labs seek ways to perform increasing numbers of cytology and histology tests with less staff, creating opportunities for suppliers of systems that can help automate both sample preparation as well as analysis. The segment is also one of the few to have benefited from significant increases in reimbursement rates, allowing labs to afford automation technologies that both improve efficiency and quality. Other new test systems introduced at the CLMA exhibition indicate that suppliers are continuing to expand the range of automation options available to labs for chemistry, immunochemistry and hematology testing, as well as for molecular diagnostics.
Rising volume, shrinking labor force
As shown in Table 1, test volumes in the clinical diagnostics industry have begun to trend upward over the past two years after a number of years of decline. Reasons for the increase include growth in hospital admissions, increasing patient acuity, expansion in reimbursement for and use of screening tests for preventative healthcare and the introduction of new therapies such as anti-HIV drugs and agents for the treatment of breast cancer that require the use of lab tests to guide drug administration. Total national spending on laboratory testing has also begun to rise, at a rate of about 5.5% per year over the past two years, after a number of years of declines. Nevertheless, spending on laboratory testing as a percentage of total U.S. healthcare expenditures has dropped to about half the level that prevailed in the early 1990s and is now at about 2.9%.
 |
In addition to growth in test volume, laboratory consolidation is another important trend that is driving demand for high-throughput automated clinical analyzers. The number of hospital labs in the U.S., based on statistics from the Center for Medicare & Medicaid Services (Baltimore, Maryland), declined 2.5% between 1996 and 2001, although the number increased 1.8% in 2001. The consolidation trend has been driven primarily by the growing domination of the healthcare provider segment by integrated health networks. According to data presented at the CLMA conference by Robert Michel, editor of The Dark Report (Lake Oswego, Oregon), there were 3,760 hospitals in 604 integrated health systems in early 1999, an 83% increase from 2,060 hospitals in integrated systems in 1995. The number of integrated health systems increased 40% over the same period. The 1999 figure represents approximately 78% of all acute care hospitals in the U.S., according to Michel. The remaining 22% are generally small hospitals of under 100 beds. In many cases, the formation of an integrated system results in consolidation of facilities, including clinical labs, in order to reduce costs. Now, with test volumes beginning to increase, the remaining labs are faced with higher test processing requirements in the face of reduced staffing, making automation mandatory.
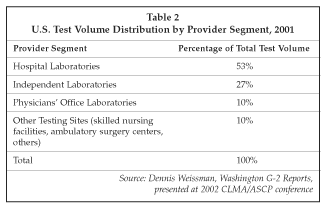
Consolidation also is a major factor in the reference lab industry. As shown in Table 2, independent labs now account for only 27% of test volume in the U.S., vs. a level of approximately 44% in 1997. The largest provider in the independent lab segment is Quest Diagnostics (Teterboro, New Jersey), with a 44% share of the independent lab segment or $4.3 billion in revenues if Unilab (Tarzana, California) is included. Labcorp (Burlington, North Carolina) is No. 2, with $2.2 billion in 2001 revenues and a 22% share. Consequently, the top two independent labs together have captured 66% of the total market. The remainder is divided among approximately 4,700 smaller testing labs. Quest acquired American Medical Laboratories (Chantilly, Virginia) earlier this year for $500 million and is in the process of acquiring Unilab for $1.1 billion.
 |
Consolidation in the independent lab market has been driven in part by increasing domination of integrated healthcare networks, which perceive value in contracting with large, full-service providers when outsourcing testing services. In addition, larger lab facilities can derive maximum benefit from high levels of automation, allowing them to reduce their cost per test relative to smaller competitors. Positive factors within the independent lab segment, according to Michel, include an increased level of discipline in managed care contracting by reference labs, vs. situations in the past in which labs were bidding contracts below cost in order to increase market share; decreased over-capacity in the industry; the increased focus on early disease detection, leading to growing test demand; and the rising volume of high-priced molecular diagnostic tests.
Manufacturers of diagnostic testing products are benefiting from the consolidation trend, the trend toward increased test volume and staffing shortages in the laboratory by offering increasingly sophisticated automated testing platforms that minimize labor requirements, reduce cost per test and allow rapid turnaround time to help maintain service levels to physicians. Leading suppliers of lab automation products, positioned to take advantage of current trends in the industry, include Roche Diagnostics (Basel, Switzerland), Beckman Coulter (Brea, California), Olympus Diagnostic Systems (Tokyo), LAB-InterLink (Omaha, Nebraska), MDS Laboratories (Brentwood, Tennessee), Bayer Diagnostics (Tarrytown, New York), Abbott Diagnostics (Abbott Park, Illinois) and Ortho Clinical Diagnostics (Raritan, New Jersey). Olympus, for example, now manufactures three systems — the OLA 1500, OLA 2500 and the OLA 4000 — for automation of specimen processing and chemistry/immunochemistry testing. The OLA 1500 performs capping, sorting and aliquotting at a rate of 1,500 tubes per hour, while the OLA 2500 adds aliquotting at rates of up to 650 tubes per hour. The OLA 4000 integrates specimen processing with chemistry and immunochemistry analysis, with analysis performed via a combination of the company's AU640 and AU2700 analyzers.
A new lab automation resource introduced by Olympus at the CLMA conference is a computer modeling program called LabModel that allows the laboratory to simulate the operation of a lab automation system prior to installation to determine if it will meet the lab's requirements. LabModel was developed in collaboration with Daniel Fink, MD, director of the central laboratory at New York-Presbyterian Hospital and Columbia Presbyterian Medical Center (New York). The core laboratory processes 5,000 to 7,000 samples per day and serves a total of 1,250 adult and pediatric beds. A key goal of the laboratory in implementing a new automated system was to decrease the variance in turnaround time. While the average turnaround time from test order receipt to result delivery was two hours, some specimens could require up to 4.5 hours for completion, resulting in physician complaints. Fink concluded that an in-depth analysis of lab processes was needed to determine where bottlenecks existed and what factors led to the variances in processing time. The LabModel simulation program allows new automation approaches to be evaluated without disrupting lab operations, and in some cases can allow evaluation of processes that are too complex to analyze in a static model. LabModel provides an alternative to the labor and time-intensive pilot program process, an expensive approach that sometimes fails to accurately simulate the operation of the actual system. Olympus will offer LabModel as a service and has added new staff dedicated to supporting the program. The typical fee for the service will be about $25,000 per project, although at present the program is still in the evaluation stage.
As the clinical laboratory industry moves toward higher levels of automation, advanced informatics systems, and expanded use of alternatives such as point-of-care testing, suppliers are adopting a variety of new strategies to remain competitive. Manufacturers of lab testing products outlined key elements of their strategies in a session covering the future of lab testing at the CLMA conference. Roche Diagnostics, for example, is launching a new web-based health information service in partnership with BioReference Labs (Elmwood Park, New Jersey). The fee-based subscription service will provide on-line training for users of Roche products, comparison of test methods offered by different suppliers, and product announcements, with content updated up to three times daily. A second aspect is to provide physician services such as physician order entry, download of test results, patient messaging, on-line purchasing of supplies, claims processing, and claims status data on-line. A third major application is disease management services, with applications for management of patients with congestive heart failure, asthma and diabetes. The service will also provide patient access to medical records.
Ortho Clinical Diagnostics, a unit of Johnson & Johnson (New Brunswick, New Jersey), is planning to develop "theranostic" products that complement its strategy of providing personalized diagnosis and treatment technologies to the market. A key focus is the development of new molecular diagnostic products for use in pharmacogenomics. An example is the hepatitis C assay now offered for guiding interferon treatment outside the U.S. The test is available for research use only within the U.S. The company said it believes that there are four times as many patients who are candidates to use the hepatitis C test as compared to viral load tests for HIV. Additional new tests will focus on guidance of therapy with drugs that are highly effective but that also have serious side effects, making it important to know if the treatment is producing the desired effect for a particular patient. Ortho also is launching new lab automation products, including the 5.1/FS chemistry/immunochemistry system, which combines chemistry, drugs of abuse, therapeutic drug, and protein testing on a single platform.
Abbott Diagnostics plans to pursue a strategy that will emphasize cancer and molecular diagnostics. The company's acquisition of Vysis (Downers Grove, Illinois) has provided a technology platform for new initiatives in those segments of the market, including molecular tests for the diagnosis of breast and bladder cancer. In addition, a recently announced alliance with Celera Diagnostics (Alameda, California) will focus on molecular tests for infectious and chronic diseases. The 250-person unit will focus initially on tests for hepatitis C, cystic fibrosis and HLA typing.
Another segment attracting growing interest within the clinical diagnostics market is products for automated cellular analysis. A number of suppliers are addressing that area as increasingly favorable reimbursement and an expanding set of clinical applications create new opportunities. CellaVision AB (Lund, Sweden), a growing supplier of automated imaging systems for cell and tissue analysis, is one of the key players in the market. In addition to the DiffMaster, a system developed by CellaVision that is used for automated white cell differential counts, the company acquired Intelligent Medical Imaging and its installed base of about 50 cell imaging systems, primarily placed in labs in the U.S. Use of the DiffMaster to perform differential counts can allow a reduction of up to 50% in hands-on time as compared to manual counting, with good agreement with manual methods. In addition, the ability to directly interface the system to the laboratory information system helps to reduce transcription errors and further reduces the labor involved in testing.
TriPath Imaging (Burlington, North Carolina), the leading supplier of automated systems for PAP smear analysis, began expanding to address other applications with the formation of TriPath Oncology in July of last year. TriPath reported 1Q02 revenues of $7.6 million, up 20% from the prior-year quarter. TriPath Oncology is a partnership that includes Millennium Pharmaceuticals (Cambridge, Massachusetts), BD (Franklin Lakes, New Jersey) and AmeriPath (Riviera Beach, Florida). AmeriPath announced an agreement earlier this year to perform validation of TriPath Oncology's development-stage Melastatin gene expression assay for malignant melanoma, which uses markers discovered by Millennium and technologies from TriPath and BD. Other development targets include assays for breast, prostate, ovarian and cervical cancer. In addition to automated imaging technology, TriPath also has developed the PrepStain Slide Processor, the FocalPoint Slide Profiler and the SurePath Test Pack. An evaluation of the PrepStain presented at the CLMA conference by Leslie Rowe, of ARUP (Salt Lake City, Utah), showed that the system, which automates Pap slide preparation, reduces turnaround time by about 45 seconds per slide. It also allows the operator to load a batch of slides and perform other tasks during processing vs. the continuous processing characteristic of manual preparation. In addition, the prepared slides contain more cells for analysis than slides prepared with other systems such as the ThinPrep Processor from Cytyc (Boxborough, Massachusetts). The PrepStain instrument is priced at $16,000, and disposables cost about $1.65 per test.
Other products for automation of cellular analysis include the Bond System from Vision Biosystems (Mount Waverley, Australia); the IQ Kinetic Slide Stainer and associated reagents from Biocare Medical (Walnut Creek, California); the Artisan system for slide preparation from CytoLogix (Cambridge, Massachusetts); the RHS1 Rapid Histo Processing Center from Hacker Instruments & Industries (Fairfield, New Jersey) and the Gemini slide stainer from Thermo Shandon (Pittsburgh, Pennsylvania). The Bond system performs both immunohistochemistry and in situ hybridization procedures in a continuous access fashion, with a 30-slide capacity. Slides are processed in batches of 10. Vision also manufactures cell analysis equipment for Leica Microsystems (Bannockburn, Illinois). The Biocare IQ system does not automate the entire procedure for preparation of immunohistochemistry slides, but does eliminate slide handling while allowing the operator to control all steps of the process. In addition, the system is attractively priced from $5,495 to $6,995, depending on the size of the waste receptacle, and eliminates consumables costs except for slides and reagents. The company also manufactures a wide range of antibodies and other ancillary equipment.
The CytoLogix Artisan system performs both special stains and immunohistochemistry tests using an automated processor. Key features of the system include individual, separately programmable heating elements for each slide, separated waste streams to facilitate toxic material disposal, 48-slide capacity and a processing time of eight minutes to over one hour, depending on the procedure. The system is priced at $63,500. More than 100 have been placed in North America since the product was launched about three years ago. The market for automated slide processing systems is being driven in part by the increasing use of new, targeted drugs that require assay of prognostic markers in order to qualify a patient for treatment. While not all markers are measured via cellular assays, drugs such as Herceptin require use of an immunohistochemical test for patient selection, and an additional 140 compounds are now in development, according to CytoLogix, that will be associated with specific targets that must be analyzed prior to beginning treatment. The company believes that a number of new applications are likely to emerge for the Artisan technology over the next few years as new drugs enter the market. Another positive factor is the recent increase in reimbursement levels for histology testing. The reimbursement for the assay technical component for special staining procedures has increased to $43 vs. a level of about $4 five years ago.
Hacker Instruments introduced the RHS1 rapid processing center at the CLMA gathering, a system that employs microwave processing to accelerate tissue fixation. The RHS-1 costs about two-thirds as much as a conventional tissue processor, yet allows same-day processing and reporting. The product uses HistoModules tailored for the specific operations required, and can process a biopsy specimen in as little as 30 minutes.
Advances in informatics drive efficiency
In addition to automation of physical processing in the clinical lab, gains in efficiency are also being realized by implementing new informatics technologies. A number of suppliers described products for automation of data management and billing at the CLMA conference. MedUnite (San Diego, California) introduced a significant advance in automated billing that provides real-time transaction capabilities for claims status inquiry, claims and encounters submission, and eligibility and benefit inquiry. The company's goal is to eliminate the need to re-enter and revise data submitted to payers because of incomplete requisitions and errors in data entry. MedUnite is funded by a consortium of seven major insurers, including Cigna Healthcare (Philadelphia, Pennsylvania), Aetna (Hartford, Connecticut), Anthem (Indianapolis, Indiana), Wellpoint (Thousand Oaks, California), Pacificare Health Systems (Santa Ana, California), Oxford Health Plans (Trumbull, Connecticut) and Health Net (Woodland Hills, California), collectively insuring 62 million members or 38% of the insured population in the U.S. The company claims that 60% of paper claims filed with insurers are not accepted on the first try, resulting in added labor costs, frustration for billing clerks (causing turnover rates of up to 40%), extended payment periods, and high bad debt expenses. For a typical claim filed for a laboratory test much of the revenue is consumed in the costs of filing the claim.
MedUnite has developed an on-line system that performs electronic eligibility checks from a physician's office via the Internet, eliminating faxes and manual labor. Since claims data is verified on-line during the filing process, the number of rejected claims is reduced and processing time is shortened. An Electronic Remittance Advice feature also provides an immediate notice of payment. MedUnite has documented a 50% reduction in Days Sales Outstanding in accounts that have adopted the system. One laboratory reduced the number of FTE positions in the billing department from three to one-half. LabCorp succeeded in recovering 5,800 claims that would have otherwise been written off by adopting the MedUnite system. The service is provided at no charge to health care providers, since MedUnite is funded by the consortium. Claims can be filed with other insurers, including Medicare, for a transaction fee of between 25 cents and 35 cents.
Major players in the U.S. laboratory information systems market include Cerner (Kansas City, Missouri), SCC Soft Computer (Palm Harbor, Florida), Orchard Software (Carmel, Indiana), Seacoast Laboratory Data Systems (Portsmouth, New Hampshire), Sysware Healthcare Systems (Farmington Hills, Michigan), McKesson HBOC (Alpharetta, Georgia), Medcom Information Systems (Hoffman Estates, Illinois), Meditech (Westwood, Massachusetts), Mediware Information Systems (Lenexa, Kansas), MISYS Healthcare Systems (Tucson, Arizona), 4Medica (Culver City, California), CCA (Calabasas, California) and Triple G Systems Group (Markham, Ontario).
Emerging suppliers include Impac Medical Systems (Mountain View, California) , SIA/Sysmex (Tucson, Arizona), LabDat (Glendale, California), MedPlus (Cincinnati, Ohio) and Atlas Medical Software/MDS (Woodland Hills, California). The industry is moving rapidly toward Internet-based systems for result reporting, including delivery of results to patients. LabDat, for example, markets a lab information system that is provided to users via an application service provider model, and that is completely Internet-based. Data can be provided to patients with a physician's permission. The company has installed four sites so far, with two more in process. Users pay either on a per-transaction basis or through a leasing arrangement.
An important focus of the LabDat system is on-line interaction with physicians and patients for explanation of test results and medical education. The company has contracted with the UPCMD consortium, a group of six medical school departments of lab medicine and pathology that includes Stanford (Palo Alto, California); the University of Southern California (Los Angeles, California), University of California, San Francisco (San Francisco, California) and Creighton University (Omaha, Nebraska), to provide an online database of information on clinical lab tests. The latest version of the system (Version 4.0, launched in mid-June), provides features such as customized pre-designation of lab ordering practices, one-click addition of patient demographic and billing information, single-page test order forms, built-in regulatory compliance and medical necessity checking. Another supplier of Internet-based lab informatics products is 4Medica, which now has installations in 50 labs nationwide. The system uses a format similar to Microsoft Outlook, providing an intuitive user interface that minimizes training requirements. 4Medica Retriever provides web-based physician access to test results, prescription ordering, and insurance authorization, including direct access to an LIS. The company provides a verification service that checks all test orders placed from a physician's office before forwarding them to the lab. Labs pay for the service through transaction fees and an initial interface fee.
Impac offers a specialty LIS focused on the oncologist. The system integrates lab data with radiology and pathology images to provide a complete electronic medical record for management of cancer patients. Impac originally developed the Multi-Access oncology-specific electronic medical record, which has been installed in 1,700 sites in 53 countries worldwide, representing a 15% to 20% share of the worldwide market for such systems. The recent acquisition of Intellidata (Woodbridge, Virginia) and its laboratory information system for small to mid-size hospitals and large group practices has allowed Impac to offer a complete solution for oncologists. The system also offers Internet-based outreach capabilities.
Major advances also are occurring in information systems for use in point-of-care testing. LifeScan/J&J (Milpitas, California) announced a new partnership with Medical Automation Systems (MAS; Charlottesville, Virginia) and Telcor (Lincoln, Nebraska) for a POC information system for management of bedside glucose testing in the hospital. LifeScan, having regained share in the whole blood glucose market following its acquisition of Inverness Medical (Waltham, Massachusetts) and the introduction of a number of new products, claims to be the market leader in hospital bedside blood glucose testing. Although Medical Automation Systems and Telcor are continuing to provide information systems for a variety of other POC testing vendors, the relationship with LifeScan provides considerably enhanced market access for both suppliers. The DataLink Information System provides remote Ethernet network connectivity for POC glucose testing, provides interfaces to a number of major lab information systems, and bi-directional data transfer from POC devices to the POC testing coordinator's workstation. More than 463 customers now are using the DataLink Connect POC data network and 89 are using DataLink Interface to transmit POC test data to an LIS. Medical Automation Systems now has installations of its POC information system in more than 400 hospitals. Users of the MAS RALS system pay a $7,000 annual license fee plus $150 per network site. In addition to LifeScan, MAS has strategic relationships with Bayer Diagnostics, Biosite Diagnostics (San Diego, California), Dade Behring (Deerfield, Illinois), Instrumentation Laboratory (Lexington, Massachusetts), i-STAT (Princeton, New Jersey), International Technidyne/Thoratec (Edison, New Jersey), Philips Medical Systems (Best, the Netherlands) and Roche Diagnostics.
Erica Klein, et al., of Providence St. Joseph Medical Center (Burbank, California), presented an evaluation of the impact of implementing automated POC data management for blood glucose testing at the CLMA conference. The group assessed time savings and clinical benefits of implementing POC data transfer using the LifeScan DataLink 3.0 system and the SureStepPro meters. The DataLink system allowed glucose results generated at the bedside to be transferred directly into the lab's MISYS LIS. Telcor implemented the interface between the DataLink and the LIS. The study found that the time required for data collection was reduced by over 90%, from 12 hours to one hour over a 72-hour period, with the added benefit of reduced patient identification errors and improved billing functions.
Bridge Medical (Solana Beach, California) is another vendor of information systems for POC data management. The Bridge MedPoint System is designed to help prevent medical errors in laboratory testing, medication administration and transfusion procedures. MedPoint is not designed to capture bedside test data, but rather to ensure that blood specimens are properly identified with the patient. The Bridge system uses pocket PCs with integrated barcode readers along with a portable printer to match test orders with the patient's bar-coded wristband and print out a label that is affixed to the sample collection container. A pocket PC also is used for tracking of blood transfusion procedures. A laptop unit is used for tracking of medication administration at the bedside. The Bridge system is the only one available that allows POC data management for all three processes (medication administration, transfusion and lab testing). The company initially focused on implementation of the medication administration system but now has one live site for transfusion tracking at Georgetown Medical Center (Washington) and is in the process of implementing the lab specimen tracking system. Bridge estimates the cost of the system for a 300-bed hospital at $400,000 to $500,000 per year for all three capabilities, plus a $200,000 implementation fee.
Point-of-care testing continues to advance
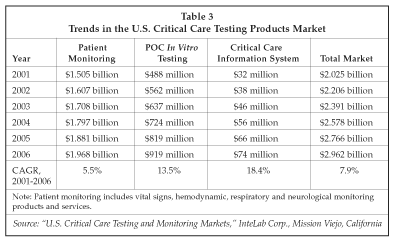
The development of effective information systems for management of point-of-care testing has helped drive continued expansion in the use of bedside testing, since one of the key hurdles to adoption of POC testing in many hospitals has been the difficulty of capturing data generated at the bedside. With systems such as the LifeScan DataLink, the DataCare system from Roche Diagnostics and POC data systems for the critical care environment such as the IDMS system from Philips Medical Systems, POC data management has become much less labor-intensive. POC information systems also have helped to resolve issues with the quality of testing performed at the point of care, particularly for blood glucose testing, by allowing labs to implement effective quality control programs that can be efficiently administered. As shown in Table 3, sales of POC testing products for use at the hospital bedside (excluding products used in stat and satellite labs) are estimated at $488 million in the U.S. in 2001, with growth forecast to average 13.5% through 2006. Growth for information systems used in the critical care setting is expected to be even higher.
 |
Radiometer Medical (Copenhagen, Denmark), one of the leading suppliers of critical care testing products worldwide, has achieved good acceptance of its NPT-7 bedside blood gas/co-oximetry testing system recently, in part due to increasing popularity of co-oximetry testing at the point of care. Another supplier planning to increase its presence in the U.S. POC blood gas testing market, Medica Corp. (Bedford, Massachusetts), exhibited its EasyStat system at the CLMA conference. The EasyStat performs blood gas/electrolyte testing at near-patient sites (although not at the bedside) at a cost of about 30 cents per sample for blood gases (70 cents per sample including electrolytes). At present, about 90% of the company's sales are to hospitals outside of the U.S., but Medica believes the EasyStat will prove popular in the U.S. market.
An additional barrier to wider adoption of POC testing has been a lack of definitive studies showing cost savings and improvement in patient outcome as a result of implementation of the technology. However, results of studies are now beginning to appear to support the benefits of POC testing. For example, a study presented at the CLMA meeting by Carol Kirchoff of the McGuire VA Hospital (Richmond, Virginia) found that the use of POC blood gas testing in respiratory therapy, using the GEM Premier system from Instrumentation Laboratory, resulted in reduced turnaround times and a considerable reduction in the time required for ventilator weaning. Test turnaround time was reduced from 34 minutes with stat lab testing to under three minutes with bedside testing. Average ventilator weaning time was reduced by 87 minutes, and some patients were weaned in under two hours vs. none in under two hours before POC testing was introduced. In addition, staff time saved by eliminating transport and clerical labor was about three hours per day and phlebotomy-related blood loss for blood gas testing was reduced by 35% to 40%. The net cost savings was estimated at more than $177,000 per year.
Because of its success in reducing costs and improving patient management with POC testing in respiratory therapy, the hospital is now planning to implement additional on-site hemostasis testing in the operating room, including expansion of the types of coagulation tests performed as well as addition of platelet function testing.
Suppliers of POC hemostasis testing products, such as Helena Laboratories (Beaumont, Texas), are responding with the introduction of new systems such as the Actalyke XL, a bedside ACT testing system cleared by the FDA in early June. The XL, priced at $3,700, provides a built-in disk drive for enhanced data management as well as an LIS interface and touch-screen operation. Cost per test ranges from $1.10 to $1.60. Helena also markets the Plateletworks system for bedside platelet function testing.
