BB&T Contributing Editor
PHOENIX – Molecular diagnostics has become a significant component of the clinical laboratory’s service offering over the past decade, due to the unique clinical utility of DNA- and RNA-based diagnostic tests, as well as to the expanding range of applications of the technology. At present, molecular testing is performed mainly by larger hospital and reference labs as well as by blood bank laboratories. Test volumes at those labs have increased in excess of three-fold over the past seven years, or at 15% to 20% a year, and the rate of increase has been even higher at 20% to 25% over the past two years, indicating an acceleration of growth in the market.
Test volume is typically dominated by infectious disease assays, which comprise 80% to 90% of the workload in most molecular diagnostic labs, but cancer testing and genetic testing also are becoming important applications, and test volumes in those areas are expanding much more rapidly than in the infection disease segment. As test volumes have grown, so has the need for automation of molecular testing. As demonstrated by the new test systems described at the 11th annual meeting of the Association for Molecular Pathology (AMP; Bethesda, Maryland), held here in early November, manufacturers have responded with a number of new automated analyzers designed to increase test throughput while reducing the labor required in molecular testing.
Advances in informatics also are enabling molecular labs to keep pace with demands for efficient and accurate processing of test requests and reporting of results. New applications such as pharmacogenetic testing have not been adopted as rapidly as initially predicted, but experts presenting at the AMP meeting believe that utilization of such tests will grow in the future.
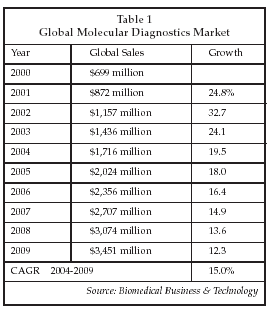
As shown in Table 1, the global market for molecular diagnostic products is estimated at over $1.7 billion for 2004, and sales are expected to approach $3.5 billion by 2009. Introduction of new test systems that enable a wider range of labs to adopt molecular testing is one factor that will drive growth in the future, as will expansion in the types of tests ordered by physicians. Market growth will be diminished somewhat by price declines for molecular testing products, as increased unit volume and competitive forces drive down average selling prices. Nevertheless, the molecular diagnostics segment is expected to remain the most rapidly growing segment of the clinical diagnostics market throughout the remainder of the decade.
Automation expanding in molecular diagnostics
Table 2 on page 10 describes new automated systems for molecular diagnostics that have recently been introduced or that are under development. Abbott Molecular Diagnostics (Des Plaines, Illinois) has established a partnership with BioGenex (San Ramon, California), one of the leading suppliers of immunohistochemistry and in situ hybridization tests, to develop a system for fluorescence in situ hybridization (FISH) automation. Test volume for FISH testing has grown substantially along with the use of Genentech’s (South San Francisco, California) Herceptin for breast cancer therapy, since testing is required in order to qualify patients for treatment based on expression of HER 2 amplification in biopsy specimens. Initially, HER 2 testing only was indicated for patients with aggressive breast cancer, but now testing is widely performed in early-stage patients to select those likely to benefit from adjuvant therapy. With over 1 million patients newly diagnosed with breast cancer worldwide each year, demand for HER 2 testing has reached a level that justifies automation in many labs.
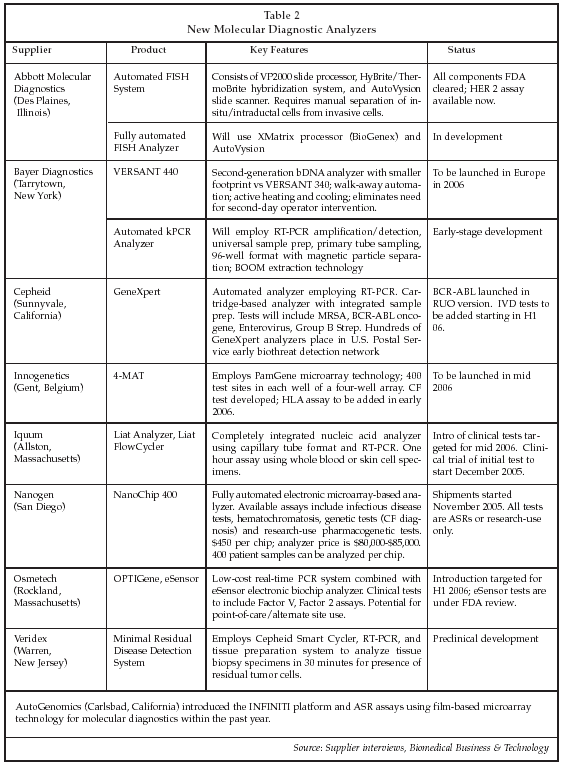
Other FISH tests that are becoming clinically important include Abbott’s UroVysion and TelVysion tests for bladder cancer and Fragile X syndrome. Goals of automation of FISH testing, based on market research conducted by Abbott of user needs, include improved consistency, higher accuracy, reduced hands-on time, higher throughput, bar-coding of samples, and random access testing. All labs polled by Abbott are forecasting high growth in FISH test volume.
Innogenetics’ (Gent, Belgium) 4MAT system is based on a unique microfluidic chip technology acquired via the company’s purchase of PamGene (S-Hertogenbosch, the Netherlands), and can automate all assay steps after sample addition. Detection is performed using a CCD chip to analyze DNA melting profiles. Up to 1,600 tests can be performed in a single run. Innogenetics has commercialized a large portfolio of infectious disease and other molecular tests in a manual line probe assay format.
Bayer Diagnostics (Tarrytown, New York) is developing a new version of its bDNA analyzer that will offer increased automation, improved sensitivity, and the ability to run both HIV and HCV assays simultaneously. For its next-generation analyzer, however, Bayer will switch to RT-PCR technology (called kPCR by Bayer) in order to achieve higher sensitivity for assays such as HIV viral load. The menu also will include tests for HCV, chlamydia, gonorrhea and HIV/HCV genotyping. Bayer will employ the BOOM extraction technology originally developed by Or-ganon Teknika (Turnhout, Belgium) and now owned by bioMerieux (Marci L’Etoile, France), but will use modified iron oxide particles with a silica nanolayer coating to provide improved extraction that is equivalent to or better than that provide by systems such as those from Qiagen (Hilden, Germany), currently the leading nucleic acid extraction and purification systems on the market. The next-generation Bayer analyzer will be capable of processing 96 specimens in less than six hours.
Nanogen (San Diego) is just beginning shipments of its new NanoChip 400 system, which is based on the company’s electronic chip microarray technology. At present, Nanogen is not marketing any molecular testing products for in vitro diagnostic use, but instead is selling the products as analyte-specific reagents (ASRs) or as research use only kits. Available ASR products include a CFTR Plus reagent for cystic fibrosis testing, a hemachromatosis ASR and an ASR for Factor V Leiden mutation. A wide range of pathogen tests is available as research-use products, as is a pharmacogenetic test panel that detects 30 different single-nucleotide polymorphisms (SNPs).
Osmetech (Rockland, Massachusetts) is developing a new molecular diagnostics platform, Optigene, that employs real-time PCR in the assay process, using low-cost plastic tubes (TOPAS tubes) developed by Osmetech. The instrument is configured to process three test cartridges, with each cartridge containing four tubes that can be independently thermocycled. The initial test menu will include assays for Factor V Leiden and Factor 2 mutations. Osmetech also is developing in vitro diagnostic tests based on the eSensor technology developed originally by Motorola (Schaumberg, Illinois) that will allow more complex assays to be offered, analyzing up to 100 markers per test.
Cepheid (Sunnyvale, California) also is planning to expand its presence in the market for in vitro diagnostic molecular tests with new assays for its GeneXpert system, which is already in widespread use for biodefense applications. The company just launched a research-use nested RT-PCR assay for the BCR-ABL mutation, used in cancer diagnosis, and will introduce an in vitro diagnostic assay for Enterovirus that will run on the GeneXpert platform in the first half of 2006, pending FDA clearance. A test for methicillin-resistant Staphylococcus aureus (MRSA) is in early-stage development that could be introduced in late 2006.
Iquum (Allston, Massachusetts) is another new entrant in the molecular diagnostic analyzer market. The company plans to initiate a clinical trial in December 2006 of a new assay for Leishmaniasis, and has developed a test platform that includes the Liat Analyzer and the Liat Flowcycler for performing semi-automated PCR-based testing.
Need for information systems grows
The growth in test volume in molecular diagnostics has not only created a need for advanced automated testing systems, but also has led to a growing demand for information systems that can process the complex results generated by molecular tests and provide reports that can be utilized effectively by clinicians. As compared to conventional laboratory tests that generate relatively simple results for a large number of parameters, the molecular lab typically processes a low to moderate volume of specimens with a large amount of data associated with each specimen. In addition, extensive data analysis often is required for each test, and reports frequently are complicated and must provide interpretative information rather than a single test value. Few commercial informatics products are available at present for managing clinical molecular diagnostics data.
As discussed by Debashis Gosh, PhD, of the University of Michigan School of Public Health (Ann Arbor), at the AMP conference, some of the existing molecular informatics programs that have been used in discovery research, such as programs using clustering analysis, have not proven effective, and may not be suitable for use in clinical data analysis. Only a few informatics vendors thus far have entered the molecular diagnostics segment, although a number of new products are in development. Cerner (Kansas City, Missouri), the leading supplier of clinical laboratory information systems, was the first to launch a molecular informatics product for the clinical lab, PathNet Helix. Helix allows genomic information generated by the lab to be stored in an electronic medical record, and also uses a structured vocabulary, known as clinical bioinformatics ontology, to present the findings. However, users must have Cerner’s core laboratory information system (LIS), Millennium, installed in order to use Helix. Gosh said that SCC Soft Computer (Palm Harbor, Florida) also is developing a new informatics product for molecular diagnostics called SoftGene, and Mysis (Raleigh, North Carolina) is considering development of such products.
A number of other laboratory informatics products have been developed by companies including GE Healthcare (Chalfont St. Giles, UK), Data Unlimited International (Gaithersburg, Maryland), Pathwork Informatics (San Jose, California) and Thermo Informatics (Woburn, Massachusetts) that can process molecular diagnostics data, but they are mainly targeted at industrial and research uses, and are not well suited to use in the clinical setting, according to Gosh. Thus there is a need in the molecular diagnostics lab for informatics products that can be integrated into any hospital’s LIS and that provide the unique capabilities demanded in molecular testing. Those capabilities include the ability to interface with a wide range of analyzers such as microarray-based systems, DNA sequencers, virtual genotyping systems and new automated molecular analyzers, as well as the ability to integrate both discrete and interpretive results in a single report.
In addition, there is a need for systems that can integrate results generated by molecular testing with information from areas such as surgical pathology and cytogenetics, since such information must often be used in concert to provide a complete diagnosis. Finally, informed consent and confidentiality capabilities are needed for clinical informatics systems that deal with genetic data in order to comply with the privacy regulations governing genetic testing.
Infectious disease testing remains lab mainstay
While new applications of molecular diagnostics in genetic testing, cancer diagnosis and pharmacogenetics are growing rapidly and expanding the role of molecular diagnostics in medicine, infectious disease testing remains the main generator of test volume at present. Testing for organisms such as chlamydia and Neisseria gonorrhoeae comprises a significant portion of overall test volume for most labs, although when viewed from a market perspective those tests constitute a diminishing percentage of the total. Other tests that now account for a large percentage of the market, estimated at 60% by suppliers, include qualitative, quantitative and genotyping assays for HIV and HCV, with HIV viral load tests accounting for 35% of the total market.
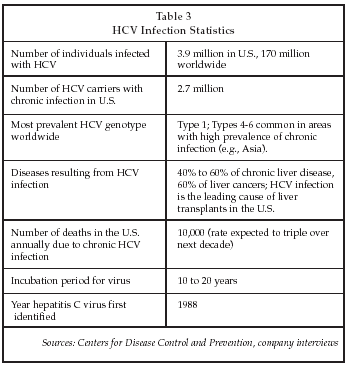
In the future, the market for HCV tests is expected to grow more rapidly than for HIV tests, and overtake that market at some point. As shown in Table 3, HCV infection constitutes a large and growing public health problem worldwide, and due to the high genetic variability of the virus and the close inter-relationship between genetic subtype and response to therapy, each new case leads to multiple HCV tests including genotyping tests.
Nucleic acid tests for HCV and HIV also are used to screen most of the blood supply in the developed countries, representing a major additional component of the market. Gen-Probe (San Diego) is the leading supplier of nucleic acid blood-screening tests worldwide. Its Procleix testing system is now used throughout most of the U.S. and Europe. Gen-Probe recently introduced the Tigris system that provides full automation of many of the company’s molecular assays, and is capable of performing 1,000 tests in 13.5 hours. The test menu includes assays for chlamydia, Neisseria gonorrhoeae, HCV and Trichomonas vaginalis as well as blood-screening assays. Tests for PCA3 (an investigational test for use as an aid in prostate cancer diagnosis) and human papilloma virus (HPV) are under development.
Products for HPV testing in particular constitute a significant component of the molecular diagnostics market, now accounting for approximately $100 million in annual sales worldwide. The only FDA-approved molecular HPV test at present is the Digene Human Papilloma Virus (HPV) marketed by Digene (Gaithersburg, Maryland). More than 99.7% of all cases of cervical cancer are associated with HPV infection. Worldwide, about 450,000 new cases of cervical cancer are diagnosed each year. HPV testing now is recommended for all women who have a Pap smear that is classified as ASCUS, i.e., that contains atypical sqaumous cell changes of undetermined significance, or for all women undergoing a Pap test who are 30 years of age or older.
The detection of HPV infection does not necessarily indicate that cervical cancer is present, since there are about 5.5 million new cases of HPV infection annually in the U.S., and about 20 million individuals harbor an infection. Most HPV infections are transient, and occur when individuals first become sexually active. In addition, only certain high-risk subtypes are linked to cervical cancer, with HPV 16 being the most prevalent cancer-associated type. Consequently, based on the most recent guidelines, screening for high-risk HPV subtypes is recommended in conjunction with most Pap smears.
Suppliers believe the market for HPV testing may potentially be as large as $500 million if screening is performed according to the latest guidelines. As a result, other suppliers, including Gen-Probe, are now developing HPV assays. The Gen-Probe test will detect viral mRNA, potentially resulting in improved sensitivity, and will test for 14 high-risk HPV types. Third Wave Technologies (Madison, Wisconsin) also has developed a group of analyte-specific reagents for use in molecular HPV typing based on its Invader assay technology.
Another infectious disease test that may represent a growth opportunity for suppliers is molecular tests for Trichomonas vaginalis, an organism that accounts for 10% to 30% of all vaginitis cases. There are more than 7 million new cases of vaginitis annually in the U.S., and an estimated 180 million individuals are affected by trichomoniasis worldwide each year. Trichomonas infection is difficult to diagnose with existing methods, since clinical symptoms (green vaginal discharge) are present in only 10% of cases. Infection is associated with low birth weight of babies born to infected mothers, pelvic inflammatory disease and urethritis in women, and prostatitis in men. A non-automated molecular test, the BD Affirm assay, has been available from BD (Franklin Lakes, New Jersey) for a number of years, but that test does not detect all cases, having a sensitivity of 80% to 90%. Gen-Probe is developing a new test for Trichomonas vaginalis that is based on the company’s TMA amplification technology. The new test exhibits 99% to 100% sensitivity when used with vaginal swab samples, or 93% if used with urine samples.
Other new opportunities for molecular tests in the infectious disease segment include new tests for sepsis as well as tests for emerging infectious agents. Improved tests for early diagnosis of sepsis represent a major clinical need. In the U.S. alone, there are about 750,000 cases annually, resulting in more than 200,000 deaths, making sepsis one of the top 10 causes of death. Furthermore, the incidence of sepsis is rising, from 82.7 per 100,000 inhabitants in 1979 to 240.4 in 2000. Worldwide, more than 18 million cases of severe sepsis occur each year.
Sepsis is a complex syndrome resulting from exposure to an infectious agent. As discussed by James Versalovic, MD, PhD, of Texas Children’s Hospital (Houston), at the AMP conference, the exact definition of sepsis remains an issue, but includes the presence of systemic inflammatory response syndrome, and acute organ dysfunction in severe cases. Both bacterial and fungal pathogens are involved, including both gram-negative and gram-positive bacteria. Sepsis resulting from fungal infections has increased more than two-fold since the 1970s. In neonates, major causes of sepsis include Group B streptococcus and Neisseria meningitis infection, while in adults infections with Staphylococcus pneumoniae and Candida albicans are common. Sepsis has proven difficult to conquer, in spite of the development of molecular tests such as the rapid Group B strep assay from Cepheid/GeneOhm Sciences, since in neonates there has been an offsetting increase in sepsis cases due to other organisms (e.g., gram-negative sepsis).
Nevertheless, the key to reducing the incidence of sepsis and lowering the associated death rate is early detection. Approximately 75% of bloodstream infections are caused by a group of 10 organisms, indicating the need for a multiplex test with high sensitivity, to allow broad-spectrum detection and initiation of therapy at an earlier stage. Molecular tests, particularly those employing amplification technology, offer the capability to detect nascent infections at a very early stage, possibly without the need for culture, or at a minimum with a considerably reduced time for culture. In addition, molecular genotyping tests can allow rapid determination of the most effective antibiotic or antifungal drug to use for treatment.
Roche Diagnostics (Indianapolis) is developing a molecular sepsis test employing PCR technology. In addition, BD is developing a 10-gene sepsis assay based on RNA expression assay technology licensed from Source MDx (Boulder, Colorado). Source MDx recently was granted a U.S. patent on the use of quantitative gene expression analysis for diagnosis of disease using two or more genes linked to inflammation or immune response, stemming from its technology for quantitative measurement of gene expression. Another test that is under development by GeneOhm Sciences (San Diego) is an RT-PCR test for direct detection of MRSA, an organism that represents a growing clinical problem in critically ill hospital patients such as those at risk for sepsis. According to Versalovic, the incidence of MRSA infection has increased 2.2-fold at his institution in the past two years. As with sepsis, early detection of MRSA infections can prove critically important in allowing effective treatment to be initiated.
Ultimately, the ideal sepsis test may include not only a panel of molecular markers for key bacteria and fungi, but may also provide an analysis of cytokine levels in the patient, since the patient’s immune response is now known to play an important role in the development of sepsis. While immunoassays for cytokine markers already are available, they are not sufficiently sensitive for use in sepsis patients, according to Versalovic, making molecular tests for cytokine RNA expression an attractive option. Patients who have inadequate cytokine responses to an infection are typically the ones who fare worst, making cytokine measurements a potentially useful tool in management of sepsis patients.
Yet another area in which molecular testing can play an important role is in the diagnosis of emerging infectious diseases. As discussed at the AMP conference by David Walker, MD, of the University of Texas Medical Branch (Galveston, Texas), more than 50 new pathogens have been identified since 1967 that cause serious disease, including viruses such as HIV, HCV, hantavirus, West Nile virus, severe acute respiratory syndrome (SARS)-associated cornavirus and ebola. New bacterial pathogens include H. pylori, the causative agent for most ulcers; the strep or staph bacteria that cause toxic shock syndrome; Rickettsia variants that cause spotted fever; and B. burgdorferi, the causative agent of Lyme disease.
Molecular diagnostics has in fact played an important role in allowing many emerging infectious diseases to be identified and characterized. Importantly, the immune response to many new infectious agents often is delayed, creating an extended window during which an infected individual may be contagious but remain undiagnosed. That problem can potentially be addressed by molecular tests that provide direct detection of the infectious agent. Examples already exist of the expansion of the molecular diagnostics market due to implementation of new tests for emerging infectious diseases, such as the blood screening test from Roche Diagnostics for West Nile virus and the Roche research-use SARS virus assay, as well as HIV and HCV nucleic acid tests.
A new application may now be emerging for molecular tests to detect the avian flu virus. As discussed by Jacqueline Katz, PhD, of the Centers for Disease Control and Prevention (Atlanta) at the AMP meeting, avian influenza has the potential to develop into a major pandemic, possibly resulting in up to 207,000 deaths in the U.S. alone. Most testing for avian flu virus now is being performed using molecular methods, mainly because classical culture techniques require early and optimal specimen collection and must be performed in a Biosafety Level 3 laboratory. The CDC has developed an RT-PCR assay for the H5 strain of the virus, and is supplying primers for use in avian flu molecular tests to public health labs along with training assistance. Based on data obtained from cases in Indonesia and China, new genetic variants of the virus are now beginning to infect humans, and Katz said she believes that the number of human infections is now increasing. Molecular testing will have a clear role in guiding public health responses to avian flu if the virus continues to spread, creating new demands for testing products.
Prodesse (Waukesha, Wisconsin) exhibited a wide range of standard-format and real-time PCR assays available as ASRs and research-use reagents for targets including West Nile virus, SARS, influenza A and B, and Bordetella pertussis, the causative agent of whooping cough. The Prodesse tests can be performed on most of the available instrument platforms used in clinical molecular labs, such as the Cepheid Smart Cycler, the iCycler IQ from Bio-Rad Laboratories (Hercules, California), and the ABI 7000 from Applied Biosystems (Foster City, California).
Expanding applications in molecular diagnostics
While infectious disease testing remains the primary application for molecular diagnostics in the clinical lab, and continues to generate new growth opportunities for suppliers, interest in other applications such as cancer testing, genetic testing, and pharmacogenetic testing is expanding rapidly. Ultimately, far more subjects are candidates for cancer, genetic, and pharmacogenetic testing than for infectious disease testing, at least barring a major worldwide infectious disease pandemic.
As shown in Table 4 below, a number of molecular diagnostic cancer tests already have been introduced, and more are in development. Gen-Probe is targeting expansion into oncology testing with a new test called PCA-3 for the detection of prostate cancer cells in urine. PCA3 is a non-coding mRNA that is over-expressed in prostate cancer cells by a factor of 60 to 100-fold. Measurement of expression levels of mRNA coding for normal PSA is used to normalize the measurement of PCA3 expression. To perform the test, urine is collected following a digital rectal exam, which results in a 60-fold increase in the number of exfoliated cells in the sample. A single cancer cell in 100 normal cells can result in elevation of the PCA3/PSA mRNA ratio above the threshold for positivity. Initial feasibility studies have demonstrated a sensitivity of 69% and specificity of up to 88%. The test may have value in improving the ability to diagnose patients with equivocal PSA immunoassay results, helping to reduce the number of unnecessary biopsies, estimated to be as high as 750,000 per year in the U.S.
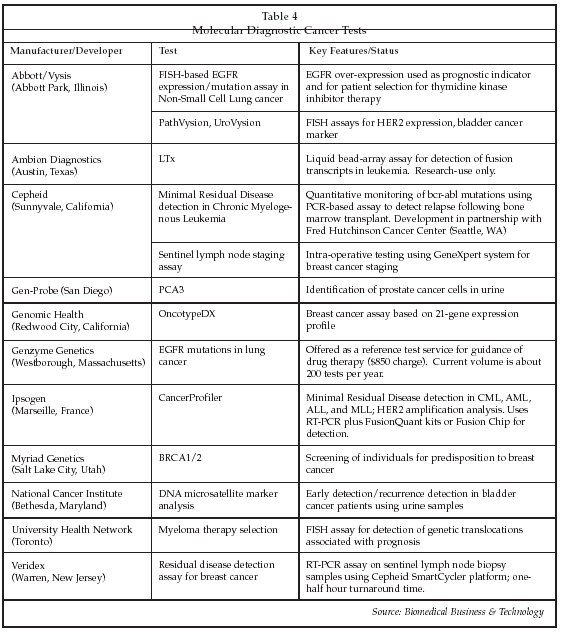
Molecular assays for epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer (NSCLC) are of considerable interest in oncology as potential tools to select patients for new targeted biological therapies with drugs such as Tarceva, an EGFR tyrosine kinase inhibitor from OSI Pharmaceuticals (Melville, New York). The drug is marketed by Genentech. EGFR is over-expressed in more than 50% of NSCLC, indicative of a poor prognosis. However, response to drugs such as Tarceva is highly variable, with about 10% of patients having an objective response, 30% to 40% having a stable response, and 40% to 50% showing progression. There are 160,000 new cases of NSCLC in the U.S. annually. Analysis of EGFR mutations using FISH probes such as those supplied by the Vysis (Downers Grove, Illinois) unit of Abbott is a promising approach to predicting which patients will respond to treatment.
Molecular tests for the bcr-abl mutation in chronic myelogenous leukemia patients also are showing promise for guidance of therapy with Gleevec, a targeted anti-cancer agent from Novartis (Basel, Switzerland). Cepheid has developed a bcr-abl kit that can be used on its GeneXpert system that allows testing to be performed close to the patient, with a two-hour turnaround time. The test, which is based on nested PCR technology, can detect one leukemia cell in 100,000 normal cells, and is performed on 100 microliters of whole blood. The test was introduced for research use in November, and the company plans a 510(k) filing early this year.
Cepheid also has developed a new assay for use in detecting tumor metastasis in breast cancer patients by measurement of molecular markers in sentinel node biopsy tissue. The test can be performed intraoperatively on the GeneXpert platform. At present, selection of the best genetic marker panel remains an issue, but the test shows a high negative predictive value and may prove useful in reducing unnecessary surgery. Attractive features of the test relative to conventional histopathology include elimination of sampling errors, since the test analyzes the complete specimen; automation; objectivity of the result; and high sensitivity.
Pharmacogenetic testing lags
Pharmacogenetic testing was another topic highlighted at the AMP conference. While some concrete and clinically valuable applications exist, pharmacogenetic testing has not yet become a major part of the workload for most molecular labs, with the exception of tests such as HER2 for patient selection for Herceptin treatment. It now appears unlikely that pharmacogenetic testing will expand to include all patients undergoing drug therapy, i.e., for the universal implementation of personalized medicine, since for many drugs the adverse effects are minimal, or the incidence of adverse drug reactions is very small, and it is consequently difficult to justify testing in order to avoid adverse reactions. It has also proven difficult to optimize drug dosage based on pharmacogenetic test results, since many factors are involved in drug metabolism. Finally, in many cases physicians already have a number of clinical parameters that can be used to predict drug response, as is the case for example with beta blockers, so there is little need for a test to predict response.
Other barriers have arisen with respect to intellectual property issues, particularly in the case of TPMT testing. Nevertheless, some success stories exist, such as HER2 testing, and the growing use of molecular hematopathology testing for patients on anti-coagulation therapy. As discussed by Roland Valdes, PhD, of the University of Louisville School of Medicine (Louisville, Kentucky), the major applications for pharmacogenetic testing likely to emerge include management of therapy with drugs that require an extended time to take effect; management of expensive therapies (e.g., Herceptin); testing to avoid adverse events for drugs that can produce irreparable harm, such as warfarin; and management of therapy with drugs that cause severe adverse reactions in susceptible patients. Major application areas cited by Valdez include anti-coagulation therapy, psychiatry, oncology, pain management, epilepsy and diabetes.
While a significant market is expected to develop in the long term for pharmacogenetic testing, the field first must overcome barriers related to insufficient associations between genotype and drug response, as well as issues with inadequate clinical interpretation, lack of availability of testing services and lack of reimbursement before the market can reach its full potential.
