BBI Contributing Editor
SALT LAKE CITY, Utah — The clinical microbiology laboratory has undergone major technology-driven change over the past decade, including advances in automation, the widespread adoption of molecular testing technologies for infectious disease diagnosis and screening, and the introduction of techniques that have allowed significant improvement in the ability to rapidly diagnose and characterize infections. The annual meeting of the American Society of Microbiology (ASM; Washington), held here in mid-May, provided a forum for discussion of both existing technologies and their impact on the microbiology lab as well as emerging technologies that will drive further evolution of infectious disease testing. As discussed by Harvey Holmes, PhD, chief of the diagnostic microbiology section at the Centers for Disease Control and Prevention (Atlanta, Georgia), at the ASM meeting, the pace of change in clinical microbiology is unprecedented. Quoting Dr. Allen Truant in a recently published text, he stated that more technology has been introduced into the clinical microbiology laboratory during the past decade than during the entire century that preceded it.
There have been significant advances in reducing the turnaround time for microbiology tests, times that were in the past so long that treatment usually started before test results were available. Now, some types of therapy, such as anti-viral drug treatment for AIDS infections, hinges on the results of viral load and viral genotyping tests in order to select the drug and dosage to be used. In other cases, the ability to rapidly and accurately diagnose a patient can allow major cost savings or improved reimbursement for the hospital. As a result, there is continued demand for automated equipment that can deliver results rapidly, requires low maintenance and minimizes labor requirements.
Emerging infectious diseases are another factor impacting the market, with more than 30 new microorganisms of clinical significance appearing since 1967, including B. Burgdorferi, West Nile virus, HIV, Neisseria meningitis and many others. In addition, antibiotic resistance is a growing issue, due both to over-use of antibiotic therapy and to the widespread use of antibiotics in the food chain. The growing mobility of the population is yet another factor contributing to the spread of infectious diseases that were once unknown outside of isolated regions of the world.
Those factors are leading to increased demand for microbiology testing, both within the clinical lab as well as in a wide range of alternate sites. New technologies, including molecular diagnostics as well as advances in automation and data analysis, have allowed labs to respond in spite of worsening staff shortages and drops in reimbursement. The consolidation of microbiology laboratories is a major trend that has created an environment conducive to the use of automated, high-volume test systems and improved cost effectiveness of testing, although lengthier turnaround times have been a drawback in some cases. In the future, experts presenting at the ASM conference expect additional advances in technology that will allow microbiologists to detect infections with increased sensitivity and shorter turnaround time, and to more accurately assess characteristics such as antibiotic and anti-viral drug resistance. Pharmacogenomics is another emerging discipline that will become important, allowing more effective drugs for infectious disease therapy to be developed and prescribed, and also allowing adverse drug reactions to be minimized.
Automation, informatics create opportunities
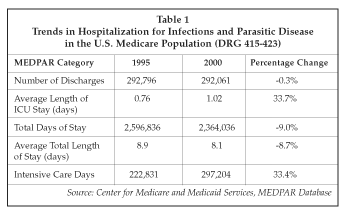
One of the key drivers of increased demand for microbiology testing in the hospital setting is greater patient acuity, due in large part to aging of the population but also to factors such the increased incidence of antibiotic resistant infections in the hospital population. As shown in Table 1, which presents data for the Medicare population over the 1995-2000 interval, there has been a 33% increase in intensive care days for patients with infectious diseases over the most recent five-year interval for which data is available. That increase occurred even though the number of hospital discharges fell slightly over the same interval. Total patient days in the hospital dropped even more, due to aggressive efforts to reduce overall length of stay, which dropped 10% from 8.9 to 8.1 days. As a result, more testing and treatment is being carried out over a shorter period of time, driving up test demand. Because many patients also are suffering from infections with multiple organisms, and in some cases, with ones that are difficult to identify, the complexity of testing is also increasing. That creates an information overload for the laboratory and for physicians that can degrade the quality of care, causing important factors impacting on patient outcome to be missed.
 |
Another source of increased demand is from patients in nursing homes and other long-term care facilities, as well as home care patients. Infection rates in long term care facilities range from 3.6 to 9.4 per thousand patients per year, which translates to 1.6 to 3.8 million cases per year, according to L.J. Strausbaugh, MD, of the Portland Veterans Affairs Medical Center (Portland, Oregon), who discussed the topic at the ASM meeting. Furthermore, the number of nursing home residents in the U.S. is projected to increase from about 2 million today to 5 million by 2030. Prophylactic antibiotics are used heavily in that setting, leading to problems with antibiotic resistance and the development of serious infections. Infections account for 26% to 50% of transfers from long-term care facilities to hospitals.
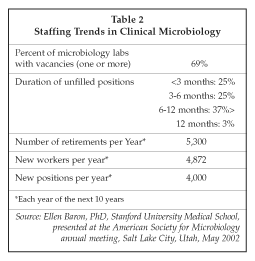
To meet such challenges, microbiology laboratories cannot simply add additional staff. As shown in Table 2 on page 179, there is a serious shortage of qualified professionals in microbiology laboratories already, and the number of new lab workers in future years is not expected to be sufficient to fill the vacancies created by retirement, much less fill the 4,000 new positions that will become available each year. As a result, labs are converting to automated systems to handle the increased workload with existing or fewer workers. Some types of conventional tests, particularly cultures to detect bacteria and viruses, are being performed less often, but new molecular tests available on semi-automated analyzers from Abbott Diagnostics (Abbott Park, Illinois), BD (Franklin Lakes, New Jersey), Roche Diagnostics (Indianapolis, Indiana), Gen-Probe (San Diego, California) and bioMerieux Vitek (St.Louis, Missouri) have more than made up for the reduction in volume. Rapid immunoassays from suppliers including Abbott, Thermo Biostar (Boulder, Colorado), Quidel (San Diego, California), OraSure Technologies (Bethlehem, Pennsylvania), Biosite Diagnostics (San Diego, California) and BD also are being used because of their ease of use, rapid (20-minute) turnaround times and relatively low cost.
 |
While newer molecular technologies have attracted the most attention, advances in conventional microbiology testing, including growth-based testing technologies as well as immunoassay methods, have produced significant benefits for the laboratory. For example, the time required to obtain results for a blood culture specimen has dropped from seven days to five days, and susceptibility testing turnaround times have dropped from 24 hours to four to six hours. The BD Bactec 9240, for example, is a fully automated blood culture system that can produce results in eight hours, vs. the semi-automated Bactec 660 that requires two days to reliably detect all organisms in the specimen. Studies described by Holmes of the CDC have shown that the fully automated Bactec allows a 15% increase in recovery of significant isolates, which translates to nine additional cases per month in a typical 550-bed hospital processing between 1,000 and 1,200 blood cultures per month. Detection of blood-borne infection (septicemia) allows a patient to be classified in a DRG having a significantly higher reimbursement level: the reimbursement for DRG 416 (septicemia) is $8,550, vs. a reimbursement of $3,024 if the patient is placed in DRG 420 (fever of unknown origin). The net result is an increase in revenue for the hospital of almost $50,000 per month.
bioMerieux Vitek also offers a line of automated blood culture systems, including the new BacTalert 3D Combination, a smaller version of the BacTalert 3D for lower-volume hospitals, priced at $42,000. Full automation for antibiotic identification and susceptibility testing is available with the $139,000 Vitek 2XL from bioMerieux Vitek. Trek Diagnostic Systems (Cleveland, Ohio) launched another new blood culture system at the ASM exhibition. Trek acquired the ESP blood culture technology previously marketed by Diffco, and has developed an enhanced version with a magnetic stirrer in each culture bottle that is used to agitate the culture broth to accelerate growth rates. The patented technique provides a shorter time to detection. The system can perform blood culture testing, mycobacterial tests and drug susceptibility tests on Mycobacterium tuberculosis cultures. A unique feature of the Trek technology is its use of pressure measurement in the head space of each blood culture bottle as an indicator of bacterial growth. Trek said it believes the technology represents a more universal approach to detection and identification of bacteria, allowing multiple types of tests to be performed in a single system. The total market for blood culture test systems and related consumables totaled an estimated $302 million in 2001.
A new approach to microbial analysis was demonstrated by Micromass UK (Manchester, UK). The MicrobeLynx System uses mass spectrometry to perform rapid speciation and typing of bacteria from a small colony. The system can analyze 96 samples in 40 minutes, and is simpler to operate than existing automated microbiology systems such as the Vitek, according to the company. List price is $139,000, plus an additional $15,000 for the database software needed for analysis of the mass spec data.
Advances in informatics also are a key trend in the microbiology lab. The field is becoming increasingly complex due to the emergence of a growing number of drug-resistant organisms, increased recognition of non-cultivable organisms and their adverse effects, the implementation of molecular diagnostic microbiology, and new requirements to test for agents of bioterrorism. To add to the complexity, many hospitals that are part of integrated delivery systems have moved their microbiology labs to a central core lab, making it more difficult for practicing clinicians to gain access to the expertise of the lab in difficult cases. Suppliers have responded by introducing new information systems such as the BD EpiCenter, which includes expert system capabilities that can aid in result interpretation; and the LabPro from Dade Behring's Microscan division (Sacramento, California). Another new system from bioMerieux Vitek, the MedMined database, is designed to help detect hospital-acquired infections at an earlier stage. Hospital-acquired infections are the fourth-leading cause of death in the U.S., and are responsible for about 80,000 deaths annually. Treatment of hospital-acquired infections also results in a non-reimbursable cost of $8,600 per case. The MedMined system provides a virtual surveillance network that extracts data from the Laboratory Information System and analyzes it to identify patterns that signal the beginning of an outbreak, generating an alert when an event is detected. Physicians review the data that triggered the alert in order to minimize false alarms. The system uses error-tolerant data processing. Hospitals pay a monthly fee starting at $5,000 for the MedMined system. bioMerieux claims that, because of the high cost of managing hospital-acquired infections, the institution saves between $4 and $8 for each $1 invested.
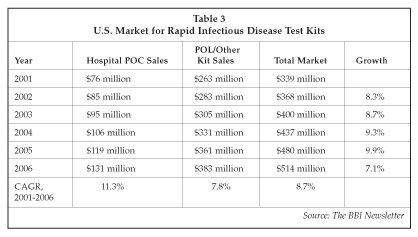
The market for rapid infectious disease test kits also has continued to attract investment. Biostar, one of the leading suppliers of rapid infectious disease immunoassays, is developing a new gonorrhea test based on the company's Optical ImmunoAssay technology that will allow detection of infection using a male urine sample, a capability that at present is only available using molecular tests. Biostar already markets a Group B Streptococcus rapid test that provides results in 20 minutes. The company expects strong sales in France, where new laws have just been passed that prevent physicians from prescribing antibiotics for certain diseases unless an infectious agent has been identified. The company also has recently launched a new rapid test for Rotavirus. As shown in Table 3, the U.S. market for rapid infectious disease tests used in the hospital and physician's office labs totaled an estimated $339 million in 2001, and growth is forecast to average 8.7% per year over the next four years, with products for hospital point-of-care (POC) use exhibiting the most rapid growth. The FDA's clearance of a number of CLIA-waived rapid infectious disease tests for POC use has helped to stimulate the market, although issues with test accuracy have raised some doubts, particularly within the Centers for Disease Control and Prevention, about the value of rapid test kits in helping to control infectious disease. The advantages of rapid tests in providing improved and more cost-effective access to testing as compared to central lab testing outweigh the disadvantages, however, helping to drive continued growth in the market.
 |
Technologies transform microbiology lab
Most of the growth in the market for products used in microbiological testing, however, is in the molecular diagnostics segment. Infectious disease testing was the first major clinical application to develop for molecular diagnostics in the clinical lab, and continues to dominate the market. Molecular diagnostics accounted for 42% of the clinical microbiology products market in 2001 (excluding infectious disease immunoassay products), and that percentage is expected to approach 50% by 2007. Applications of molecular diagnostics in clinical microbiology include more rapid and definitive identification of organisms than is possible using culture methods, screening for and diagnosis of chlamydia and gonorrhea infections, HIV and HCV viral load testing, viral genotyping to guide drug therapy, and monitoring for cytomegalovirus infection in transplant patients. Real-time PCR, for example, provides a fundamentally improved approach for identifying organisms responsible for outbreaks of infection in the hospital. As discussed by F.R. Cockerill III, MD, of the Mayo Clinic (Rochester, Minnesota), at the ASM conference, the use of culture methods for identification requires an initial incubation of at least 24 hours, followed by a purification step and subculture requiring another 18 to 24 hours, and a third 24-hour culture step to determine drug susceptibility, for a total time of three days minimum. Using real-time PCR performed with systems such as the LightCycler from Roche Diagnostics, results can be available within one day with equivalent sensitivity and in some cases improved discrimination. At a minimum, turnaround times for screening for methicillin-resistant S. aureus or vancomycin-resistant enterococcus can be reduced by one day. In the case of viral diagnosis, the differences in turnaround time can be even more profound. Detection of enterovirus, for example, can be performed in four to five hours with molecular methods vs. seven to 10 days using culture methods. In Cockerill's opinion, culture methods are no longer acceptable for use in infection control in the hospital, given the alternative of performing real-time PCR.
Monitoring of organ transplant patients for the development of CMV infections is now a standard of care in transplant centers. Molecular methods have proven to be a major advance in that application. Available techniques include nucleic acid amplification methods such as NASBA from Organon Teknika/bioMerieux; antigen testing via immunoassay methods; PCR; the Digene (Gaithersburg, Maryland) Hybrid Capture assay technology; and mRNA assays that detect the message coding for the p67 antigen associated with the virus. The cost of such test methods now is less than $25 per test (typically one test per week is performed), vs. the cost for antibiotic prophylaxis of thousands of dollars per week. Molecular tests can now detect the development of an infection well before clinical symptoms appear, allowing prolonged courses of anti-viral drug therapy to be avoided, not only reducing cost but also avoiding the early onset of drug resistance. Similar examples exist for HIV viral load testing and HIV genotyping in AIDS patients. An HIV viral load test costing $100 performed at two- to three-month intervals can save thousands of dollars in costs related to ineffective drug treatment and management of associated sequelae, as can HIV genotyping tests costing $350 to $450.
bioMerieux Vitek is one leading supplier of conventional microbiology testing systems that is now developing molecular testing technologies for the clinical lab. bioMerieux acquired Organon Teknika from Akzo Nobel (Arnhem, the Netherlands) for Euro 311 million in mid-2001, and has since initiated development of a number of new clinical tests based on the NASBA nucleic acid amplification technology. Research-use tests already are available for CMV and HIV-1 that use the Origen electrochemiluminescence labeling technology licensed from Igen (Gaithersburg, Maryland). However, bioMerieux plans to use a new technology, molecular beacons, in its clinical tests, which will allow real-time read-out. Molecular beacons use a fluorophore and a fluorescence quencher at opposite ends of a hairpin nucleic acid probe. Fluorescence is quenched until the probe binds to its target because of the hairpin structure of the unbound probe. Upon binding, the signal is enhanced 200- to 300-fold. Fluorophores emitting at different wavelengths can be used for various probes to perform multiplexed assays. Up to four assays have been performed in a single, closed tube using molecular beacons, and up to six are theoretically possible. The new bioMerieux line, called Easy Q, will have higher throughput than the research-use tests now available, as well as greater ease of use. Easy Q tests using molecular beacon labels are now undergoing FDA review. When cleared for marketing, tests such as HIV-1 will sell for $50 to $60. A test system will be available that will include the EasyQ Analyzer, the EasyQ Incubator and the NucliSens Extractor for automated isolation of nucleic acids (RNA).
bioMerieux also is developing molecular assays for its Vidas Probe System employing the Transcription Mediated Amplification technology from Gen-Probe. Those tests can be performed on the existing Vidas immunoassay instrument, with an installed base numbering about 14,000 worldwide. However, nucleic acid probe tests for the Vidas are not yet available in the U.S. Gen-Probe and bioMerieux are also collaborating in the development of Tigris, a fully automated molecular diagnostics system that is in beta trials this year. Clinical trials with Tigris are planned for 2003. Tests will include a combination chlamydia/gonorrhea assay, and an amplified Mycobacterium tuberculosis test. All processing of the sample will be performed in the primary specimen tube, and the system will have the capability to generate 2,000 results on 1,000 specimens in 12 hours. Another application is detection of human papilloma virus subtypes in PAP specimens, specifically using the ThinPrep specimen preparation technology now marketed by Cytyc (Boxborough, Massachusetts).
Digene, now in the process of being acquired by Cytyc for $554 million, already sells HPV assays for use with the ThinPrep specimens. In fact, 80% of the tests performed using the Digene kits are for use with ThinPrep samples, according to Digene. The company has also just submitted a new HPV kit for FDA clearance that can be performed using the SurePap Liquid-Based Cytology Slide from TriPath Imaging (Burlington, North Carolina), the leading supplier of automated image analysis systems for use in the analysis of Pap smears. Another application developed by Digene, screening for chlamydia and gonorrhea via molecular diagnostic techniques, was cleared for marketing by the FDA more than a year ago. However, there have been no placements of the Digene Rapid Capture System for CT/GC testing because an alliance between Digene and Abbott to market the system was unsuccessful. In early June, Cytyc said that it would enter the market for chlamydia and gonorrhea screening with a new test just approved by the FDA that will allow testing directly from the ThinPrep vial using the Cobas Amplicor system from Roche Diagnostics.
Roche is the largest player in the molecular infectious disease diagnostic products market at present, with more than 4,000 Cobas Amplicor systems placed since 1995. Roche has continued to invest in new molecular testing technologies to complement its fundamental PCR technology, which is by far the most widely used nucleic acid target amplification methodology. One of the new offerings now under development is a series of infectious disease Analyte Specific Reagent (ASR) products that will run on the Roche LightCycler, a real-time PCR system targeted primarily at the research market until recently. Many molecular diagnostics labs now use the LightCycler to perform tests developed in-house, along with the Roche MagnaPure nucleic acid extraction system. Eleven new ASR LightCycler tests are under development, including HSV-1/2, VRE, MRSE, and Group A and B streptococcus. The system will be capable of processing 32 samples in 30-90 minutes, depending on the sample preparation protocol. List price for the LightCycler is $57,500, and the price for the MagnaPure system is $84,500. More than 3,000 LightCycler instruments are installed worldwide, including approximately 1,000 in the U.S. Roche also is planning to expand into HPV testing following its acquisition of the rights to HPV patents from the Institut Pasteur (Paris).
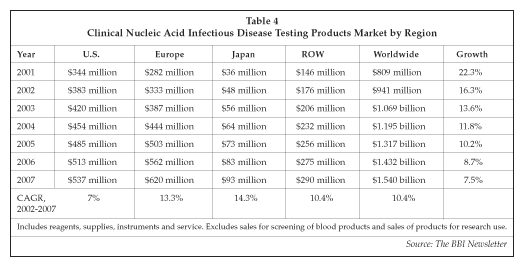
The companies in the clinical molecular diagnostics market are addressing a large and growing opportunity, as shown in Table 4. The global market for molecular infectious disease testing products is forecast to exceed $1 billion next year, although growth is expected to slow over the next few years as the market begins to mature. However, other segments, including products for oncology and genetic testing as well as pharmacogenomic tests, are now beginning to enter a rapid growth phase, providing opportunities for continued expansion for suppliers.
 |
One avenue for technological advances in the molecular diagnostics arena is the development of even more sensitive and rapid methods for detection and identification of infectious agents. One of the limitations to existing approaches such as PCR-based assays in detecting early-stage infections is the inability of such methods to detect all organisms of interest. As discussed by Dr. David Relman of Stanford University (Paolo Alto, California) at the ASM conference, the universal primers used to detect infectious organisms typically are not truly universal, and thus miss some types. In addition, studies with very sensitive techniques have shown that nucleic acid sequences from pathogenic organisms can be detected in blood and cerebrospinal fluid in states of health, creating a background that complicates test interpretation. The timing, anatomic location and volume of specimen collection also create considerable variability when attempting to isolate an infectious agent from a patient. As a result, existing techniques can exhibit quite low sensitivity, from 50% to 70% in pneumonia and 30% in encephalitis to as low as 10% in sepsis. Relman is investigating a new method that analyzes host mRNA expression profiles using nucleic acid microarray analysis and pattern recognition techniques. The method is analogous to assessing immune response to detect infection except that nucleic acids are analyzed rather than antibody levels. He has found that changes in expression occur within minutes after exposure to an agent, indicating the modality could potentially be used to rapidly detect infection outbreaks in the hospital or for bioterrorism applications. In addition, a dose-response relationship is observed, although universally shared relationships among patients are not apparent. So far, studies analyzing the response levels of 67 genes have shown that the variability is increased about eight-fold in disease states vs. normals. The technique has been studied for use in identifying the causative agent in fever epidemics in Nepal, as well as for early detection of cancer. Importantly, expression patterns can be correlated with the type of organism causing the infection, with one study showing the ability to identify Salmonella as the causative agent with a sensitivity of 80%.
Another new technology that promises to significantly reduce turnaround time for molecular infectious disease tests is under development by Cepheid (Sunnyvale, California). Cepheid's long-range goal is to develop a near-patient molecular diagnostic system that provides automated sample preparation, turnaround times of under 30 minutes (vs. more than five hours for some existing systems), and high detection sensitivity. The Cepheid GeneXpert system is to enter beta testing before the end of the year, with launch planned in 2003. It will use molecular beacon labels and homogeneous assay techniques whenever possible. A control tube will be run along with each sample to ensure result validity, and all reagents will be supplied in lyophilized form sealed in the test cartridge. Specimen volumes can range from 5 microliters up to 100 milliliters, the latter being important when attempting to analyze food or environmental samples or to detect targets that are at extremely low concentrations. An integrated ultrasonic transducer is included for lysis of cells and disruption of particulates. Cepheid said it believes the system design will meet the requirements for CLIA-waived status. The cartridge will cost between $15 and $100, depending on the test. Key applications include detection of sepsis, meningitis, and biothreat agent exposure; infection control in the hospital; and rapid diagnosis of infections in outpatients. A Group B streptococcus test is under development that has a sensitivity of 97% (vs. the FDA's hurdle of 80%) and that will cost about $20 per test.
Another unique approach to improving the sensitivity of detection of infectious agents is being developed at the Massachusetts Institute of Technology Lincoln Laboratory (Lexington, Massachusetts) by a group led by Dr. M. Petrovick. The MIT group is investigating cell and tissue-based sensors for use in infectious disease testing that consist of cells plated on electrodes. The electrical potentials generated by each cell in response to exposure to known agents are recorded. Subsequent exposure to the agent allows rapid detection and identification, with a typical turnaround time of 15 minutes. An advantage vs. molecular techniques is that the cell responds to all agents affecting metabolism, not just ones that contain a matching nucleic acid sequence. In studies to evaluate detection of bacteria and viruses, the Canary B-Cell Sensor was able to detect as little as 50 colony-forming units in under three minutes.
A new system for accelerating the rate of PCR reactions was exhibited at the ASM meeting by Alpha Helix (Uppsala, Sweden). The Super Convector uses g-forces (from centrifugation) to create turbulence in the PCR reaction tube, which not only provides instantaneous mixing but also instant temperature changes. A complete 35-cycle PCR incubation can be completed in 12 minutes. A laser excitation and detection system is used for real-time detection at seven different wavelengths, providing multiplexing capabilities. An infrared lamp is used for heating, and refrigerated air is used for cooling. The system is in beta trials, with launch for research applications planned in 2003.
