CDU Contributing Editor
BALTIMORE, Maryland – The 27th annual scientific meeting of the Society of Cardiovascular & Interventional Radiology (SCVIR; Fairfax, Virginia), held here in April, provided an update on new advances in the diverse field of interventional radiology. Some of the most significant recent breakthroughs in transcatheter intervention, such as drug-eluting stents and endovascular stent-grafts for aneurysm repair, were highlighted at this year's conference. Continued improvements in diagnostic imaging as well as enhanced technologies for catheter or needle guidance have increased the safety of procedures and the ability of physicians to convert surgical procedures to less-invasive transcatheter procedures. Advances also were described in the areas of carotid stents, intracerebral stents, and embolization protection devices for use in neurovascular interventions.
Embolization therapy continues to advance, with the introduction of new devices such as the MicruSphere from Micrus (Mountain View, California) and EmboGold microspheres from Biosphere Medical (Rockland, Massachusetts), and the almost complete replacement of some types of surgical aneurysm treatments by less-invasive methods. Dialysis access is another important application area for interventional radiologists, and one that is growing rapidly as the number of dialysis patients increases and new technologies to improve access patency and ease of use are introduced. Important applications, including vertebroplasty and varicose vein therapy, continue to expand as interventional radiologists become more familiar with new technologies and patient awareness of the availability and effectiveness of those procedures increases. One important new product exhibited at SCVIR was the ELVS Endovascular Laser Venous System from AngioDynamics (Queensbury, New York), which is awaiting 510(k) clearance for endovascular treatment of varicose veins, addressing a patient population comprising 25% of all women and 15% of men worldwide, including 80 million people in the U.S.
However, the field of interventional radiology continues to face a number of issues, including lack of recognition by the general public, difficulties in obtaining adequate reimbursement for procedures, and shortages of trained personnel. Cardiologists are capturing a growing percentage of interventional radiology procedures, as are vascular surgeons. Nevertheless, suppliers continue to invest in new technologies for interventional radiology, and to bring new products to the market. Market demand is expected to expand due to a variety of factors, including continued increases in the prevalence of diabetes, the growing prevalence of obesity in the U.S., and aging of the population. An estimated 8 million people in the U.S. have peripheral vascular disease, a condition that becomes more prevalent with age, and which is increasingly being treated using interventional radiology techniques.
Expansion in peripheral stent market
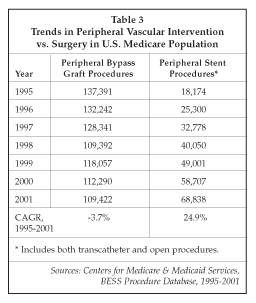
Peripheral vascular stents have been identified as a key new growth segment by many of the existing suppliers of coronary stents. As shown in Table 3, the number of peripheral vascular stent procedures performed in the U.S. increased at a compound annual rate of almost 25% over the 1995-2001 period, while peripheral vascular bypass surgery procedures declined, indicating some replacement of surgical treatment with interventional therapy. Over the same period, use of another key vascular surgery procedure, creation of a dialysis shunt, has increased markedly. That procedure, while not at risk of being replaced by minimally invasive therapy, is helping to drive growth in demand for peripheral vascular angioplasty products and stents since physicians have found that the use of stents in dialysis shunts can help prolong patency. Peripheral vascular stenting may be poised to enter a new phase of rapid growth as advances in stent design, including drug-eluting stents and stents designed to withstand the mechanical stresses encountered in sites such as the carotid arteries and vessels in the legs, become available for clinical use.
 |
The results of the SIROCCO trial using a drug-eluting S.M.A.R.T. stent from the Cordis (Miami Lakes, Florida) unit of Johnson & Johnson (New Brunswick, New Jersey) were presented at the SCVIR gathering by Vincent Oliva, MD, of Hopital Notre Dame (Paris). The stents used in the trial included a coating containing the Sirolimus drug as used in the Cordis Cypher coronary stent, and were implanted in the superficial femoral artery (SFA) in 33 patients in five centers in Europe and one in Canada in a feasibility study. All patients were evaluated using quantitative angiography and Duplex ultrasound at six months after the implant procedure. The study was especially challenging because 100% of the patients in the stent group had calcified lesions vs. only 47% in the control group, and 57% had chronic total occlusions. Average lesion length in the treatment group was also quite high at 8.5 cm. Consequently, the patients are representative of some of the more difficult cases that would be encountered in routine clinical practice.
The drug-eluting, self-expandable S.M.A.R.T. stent completely eliminated restenosis, whereas 23.5% of the patients in the control group had restenosis. Not only did the use of Sirolimus provide an obvious benefit, but the S.M.A.R.T. stent itself also performed well, since typical restenosis rates in the SFA using existing treatment techniques are around 70%. However, an adverse finding was that stress fractures occurred in the stent struts in six of the 33 patients, all in patients who had three or more stents implanted. While no adverse clinical events have occurred so far that were related to the fractures, there is a question about possible long-term failure, as well as about the stance that regulatory agencies such as the FDA will take on granting marketing clearance given the unknown consequences of the fractures on long-term patient outcome. Clearly, it would be preferable to switch to a stent design that does not exhibit fracture problems, assuming other performance aspects would not be affected. In any case, the results of the study indicate that drug-eluting stents may allow successful transcatheter treatment of blockages in small-diameter peripheral vessels that until now were not believed to be appropriate for stenting. Based on surgical bypass procedure data, patients with lesions in the smaller-diameter vessels of the leg may comprise 75% of all patients requiring peripheral revascularization.
Another approach to using drugs to inhibit or prevent restenosis in peripheral vascular stent procedures was described by Matthew Johnson, MD, of Roudebush Veterans Affairs Medical Center (Indianapolis, Indiana) at the SCVIR sessions. Johnson has used peri-adventitial drug delivery in explanted vessels using agents including paclitaxel and demonstrated that high local drug concentrations can be achieved in the adventitia of a vessel, resulting in elimination of intimal hyperplasia. Delivery of drug to the adventitia, in principle, avoids the washout of drug associated with intravascular delivery. Some other approaches to delivering drugs for restenosis prevention have not produced as promising results. While some early clinical success was achieved with the Igaki-Tamai bioabsorbable stent, more recent studies with a poly-L-lactic acid stent loaded with the anti-proliferative drug carcumin, while demonstrating a reduction in proliferation, also showed that the stent material has an inherent inflammatory effect.
Other studies using paclitaxel-eluting stents were described by Lindsay Machan, MD, of University Hospital (Vancouver, British Columbia). Machan has been involved with the development of paclitaxel-eluting stents in collaboration with Angiotech Pharmaceuticals (also Vancouver) for more than eight years, and has studied mechanisms of action of the drug in conjunction with interventional therapy in detail. Although paclitaxel is a potent chemotherapy agent when administered in high doses, its action can be quite different depending on the dosage used. The drug has been shown to inhibit inflammation, smooth muscle cell proliferation and secretion of extracellular matrix, all factors in the development of restenosis. The most recent studies, conducted with paclitaxel-eluting coronary stents under development by Boston Scientific (Natick, Massachusetts), have demonstrated complete inhibition of restenosis at six months. According to Machan, one of the most challenging aspects of development of the paclitaxel-eluting stent was the design of the coating used as a drug reservoir on the stent. The strong dose dependence of the effects of paclitaxel clearly requires that the coating allow precise and reliable control of drug elution, while also tolerating the expansion associated with stent deployment. At present, the Boston Scientific device appears to be the best candidate to compete with the Cordis drug-eluting S.M.A.R.T. stent in peripheral vascular applications, although no studies have yet been described by Boston Scientific using peripheral vascular drug-eluting stents.
Numerous suppliers are developing stents without drug-eluting capabilities for applications in the peripheral vessels. Sulzer IntraTherapeutics, a subsidiary of Sulzer Medica (Zurich, Switzerland), announced FDA approval of the IntraCoil self-expanding peripheral stent at the SCVIR conference. The IntraCoil is approved for use in the treatment of occlusive disease in the femoral and popliteal arteries, and is the first stent to be approved by the FDA for use in those vessels, and also the first non-coronary stent to be approved for use as a primary treatment. About 15% of the eight million individuals with peripheral vascular disease in the U.S. have blockages in their femoral or popliteal arteries. Sulzer IntraTherapeutics has a broad line of peripheral stents, including the Prot g , IntraStent, and DoubleStrut biliary stents, the Paramount stent and the IntraCoil self-expanding bronchial nitinol stent.
Other new developments in peripheral vascular stents were announced by C.R. Bard (Billerica, Massachusetts), Cook (Bloomington, Indiana), Medtronic (Minneapolis, Minnesota) and AngioDynamics. Bard introduced a new version of its Luminexx self-expanding nitinol biliary stent that allows delivery of devices having expanded diameters of up to 12 mm via a 6 Fr delivery system, which will compete with the Boston Scientific Wallstent, also offered in a 6 Fr version. Bard sold about 15,000 Luminexx stents in 2001, and expects to gain additional market share as a result of the introduction of the 6 Fr Luminexx. The company expects additional competition in that segment with the pending launch of a new version of the Cordis Precise biliary stent having expanded diameters up to 10 mm coupled with a 6 Fr delivery system, but that product is at least four months away, according to Bard. Cook has introduced the Zilver self-expanding biliary stent, using a 7 Fr delivery system. The Zilver uses Cook's Z-stent cell design, and exhibits no foreshortening on deployment, allowing more accurate placement. Medtronic AVE (Santa Rosa, California) announced the launch of the Assurant balloon-expandable biliary stent, which can be delivered via a 6 Fr sheath, and will complement its Bridge SE self-expanding biliary stent. AngioDynamics introduced the OmniFlex 15 mm biliary stent, a new device that, in addition to offering a large expanded diameter, is fabricated from a single platinum wire, reducing foreshortening. The platinum stent also offers the advantage of invisibility in magnetic resonance images but high visibility on fluoroscopic images.
Other stents under development for the U.S. market exhibited at the SCVIR conference include the SelfX self-expanding biliary stent from Jomed AB (Helsingborg, Sweden) and the Niti-S line of self-expandable stents from Taewoong Medical (Seoul, South Korea). Taewoong's line includes stents for vascular, biliary, esophageal, gastrointestinal, TIPS and colorectal use. The Taewoong colorectal stent, intended for use in palliative therapy in cancer patients, is available in a covered, retrievable version. A new approach to stent design was described by IDev Technologies (Houston, Texas) that uses nitinol wires or wire pairs to fabricate stretchable stents. The company also has designed stretchable caval filters and occlusion devices. The stretchable design results in a lower delivery profile while providing a large deployed diameter.
Another technology that should help to expand the use of stents in the treatment of peripheral vascular occlusive disease has been developed by TransVascular (Menlo Park, California). The company received 510(k) clearance for its CrossPoint TransAccess catheter on April 8. The CrossPoint is intended for use in conjunction with interventional procedures employing a guidewire to open occluded peripheral vessels prior to angioplasty or stenting. In some cases, the guidewire becomes trapped in the wall of the vessel, causing the procedure to be aborted in favor of surgical repair. The CrossPoint catheter helps return the guidewire to the true lumen beyond the blockage, allowing treatment to be completed without resorting to surgical bypass. TransVascular also is developing devices for performing transcatheter coronary artery bypass.
One of the trends in the peripheral stent sector in recent years is increasing off-label use of the devices. That has been a major factor driving expansion of the market since, until recently, essentially all devices were only approved for biliary use, at least in the U.S. As discussed by Elisa Harvey, DVM, PhD, of the FDA's Center for Devices and Radiological Health (Rockville, Maryland), there has been an exponential rise in the number of biliary stents cleared for marketing, including 29 in 2001 alone. Another five devices were cleared in the first quarter of this year. According to Harvey, the number of devices implanted far exceeds the number of available patients if only the approved device indication (palliation of malignant biliary strictures) is considered. Clearly, physicians are using the devices off-label for a variety of other applications, since the dimensions for biliary stents overlap with those needed for coronary, carotid, and a number of other types of peripheral vascular stents. The reasons for that trend are obvious, since obtaining device labeling for other vascular applications requires premarket approval. However, the practice raises an issue regarding the widespread use of permanent implants without adequate safety and efficacy data pertaining to the actual mode of use. For example, stents used in the SFA or the carotid artery are subjected to different mechanical stresses than are biliary stents, but there are no requirements to test a device's ability to withstand such stress if it is approved only for biliary indications.
Some suppliers are responding by undertaking clinical trials to obtain the data needed for approval for the actual site of use. For example, Medtronic is conducting studies in other body sites using its peripheral vascular stents, as is Cordis. In principle, suppliers obtaining such approvals would have an advantage, since the physician and the hospital would not be assuming the responsibility and risk associated with off-label use. Such trials can be difficult to perform, however, since many patients who are eligible for the trial opt for off-label treatment with an existing device. As more stents are approved for specific indications, the market will likely begin to favor suppliers offering devices that are labeled for a particular use, since liability issues could emerge for physicians with off-label use once a device is available that has the appropriate approvals.
One of the most significant emerging opportunities is stents for treating cerebrovascular disease, including carotid stents as well as intracerebral stents. There has been an on-going debate about the merits of carotid stenting vs. surgery (carotid endarterectomy), as well as development delays while embolization protection devices are perfected. While some widely-quoted studies such as the NASCET trial have shown very low (0.6%) rates of death for surgery, advocates of carotid stenting, such as J.J. (Buddy) Connors, MD, of Inova Fairfax Hospital (Falls Church, Virginia), point to data from individual hospitals showing death rates of 2% to 2.5%, making the procedure one of the most dangerous surgeries performed in most hospitals. Carotid stenting has emerged as an alternative to surgery, with about 9,000 patients now treated worldwide in investigational procedures, according to a survey of 49 medical centers conducted by Mark Wholey, MD, of Pittsburgh Vascular Institute (Pittsburgh, Pennsylvania). Procedure volume is increasing at 35% per year. The majority (about 54%) of the procedures have used Wallstents. Death and complication rates are very similar to those reported for carotid endarterectomy (procedural mortality in the survey population is 0.7%). Only 13% of all cases used an embolic protection device, since the survey included cases dating to 1997.
Other stents under development for carotid use include the Cordis S.M.A.R.T., the Accu-Link from Guidant (Indianapolis, Indiana), the Nexstent from Boston Scientific, the Exact from MedNova Ltd. (Galway, Ireland) and a nitinol stent from Medtronic. The devices are being evaluated in three major carotid stent trials or registries: SAPPHIRE, CARESS and CREST. The CREST trial is on hold after accruing 130 cases due to problems with Guidant's AccuNet embolic protection device used in that trial. About 70 patients had been entered into the CARESS trial as of early April 2002. A total of 300 patients who have received implants of the Cordis S.M.A.R.T. stent have been entered into the SAPPHIRE registry so far. A variety of protection devices are also being evaluated for use in carotid stent procedures, including the Medtronic/PercuSurge GuardWire; the Cordis AngioGuard filter; the Boston Scientific EPI Filterwire, the MedNova Neuroshield; the TRAP from Microvena (White Bear Lake, Minnesota), the E-Trap from Metamorphic Surgical Devices (Pittsburgh, Pennsylvania) and the Parodi catheter from ArteriA (San Francisco, California).
The market opportunity for cerebrovascular stents may be considerably larger than indicated by the number of carotid endarterectomy procedures performed annually. As discussed by Connors at an SCVIR press conference, stents can also play a role in prevention if patients are diagnosed early with carotid stenosis or stenosis of other cerebrovascular vessels. Patients with intracranial stenosis have a 15% risk of suffering a stroke within a year, according to Connors, but are rarely treated because of the risks associated with surgery and lack of awareness of stenting as an alternative. In comparison, intracranial aneurysms and arterio-venous malformations, two conditions that generally elicit major concern among patients and are usually treated aggressively, carry annual stroke risks of 1% and 2% to 4% per year; respectively. Connors said he believes that, ultimately, 95% of intracranial stenting will be performed as a preventive measure. About 40,000 strokes occur each year due to intracranial stenosis, and many could likely be prevented using stents. Although there are as yet no stents approved for intracranial or carotid use in the U.S., devices are under development, including drug-eluting versions for use in smaller intracranial vessels where restenosis is an issue. Medtronic has launched a new stent, the INX, in Europe for the treatment of brain aneurysms.
Endovascular grafts a growth sector
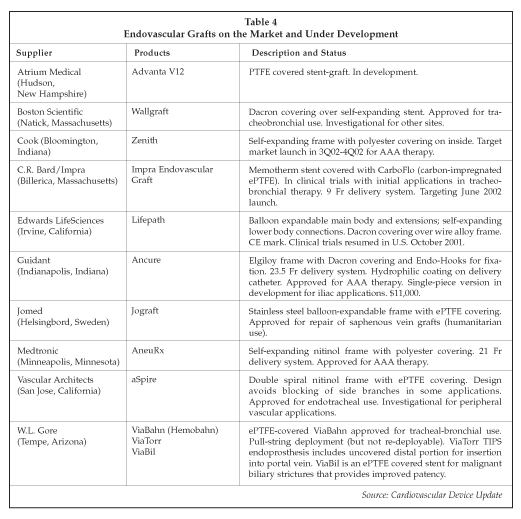
Another major growth segment in interventional radiology is endovascular grafts for the treatment of aneurysms. Guidant and Medtronic introduced the first devices in the U.S. market in 1999. Now, some physicians treat 80% of the patients presenting with abdominal aortic aneurysm using endovascular grafts. Medtronic claims the leading position in the market with the AneuRx stent-graft, with more than 20,000 implants worldwide. Guidant is No. 2, with approximately 12,000 Ancure devices implanted worldwide. The Ancure unit price is about $11,000. Guidant pulled its device off the market for part of 2001 to implement some modifications to the delivery catheter, but is now actively selling the Ancure worldwide. In 2001, Ancure sales totaled $45.6 million, corresponding to seven months of sales, vs. sales of $62.7 million in 2000 when the Ancure was on the market for a full 12 months. Endovascular graft products on the market and under development are described in Table 4.
 |
Endoleaks, occurring in 10% to 20% of patients, continue to be an issue for all of the available devices, as is cost. A study presented by Watson et al., of Riverside Methodist Hospital (Columbus, Ohio) comparing costs for open surgical repair to endovascular repair found that surgery generated a profit of $1,566 per procedure while endovascular repair resulted in a loss of $8,818. The same study found a 46% rate of endoleaks for endovascular repair vs. 0% for surgery, although length of stay in the hospital and blood loss were both reduced for endovascular repair. The study did not factor in savings of endovascular repair due to faster return to work, or additional costs for post-procedure imaging and catheterization. In Europe, suppliers are finding that physicians are now more more selective in performing implant procedures dues to concerns about cost and endoleaks or other adverse events. In addition, in about 5% of patients, aneurysms continue to grow even though no endoleaks are detectable, although conversely, the devices are effective in either reducing aneurysm size or preventing further growth in 95% of patients.
In spite of performance and cost issues, however, the use of endovascular grafts for AAA repair and other applications is growing rapidly. Patients often prefer non-surgical treatment, and continued improvements in device performance are making procedures safer and more effective. As discussed by Gary Becker, MD, of Miami Cardiac & Vascular Institute (Miami, Florida), at SCVIR, there is a learning curve associated with performing endovascular graft procedures that can affect costs when the technique is first adopted. After approximately 100 procedures were performed at Becker's institution, corresponding to an average of about 20 procedures per interventionalist, the procedure time dropped considerably to about 150 minutes. Becker's institution now performs about 100 cases of open surgery per year (vs. 10 per year eight years ago), and about 300 endovascular graft cases have been performed in the past four years. Endovascular repair is not offered to patients under the age of 67 due to concerns about the long-term performance of endovascular grafts.
Growing opportunities in dialysis access
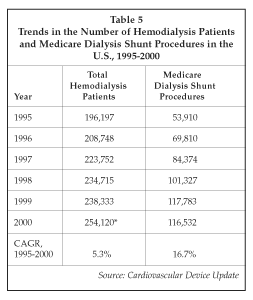
Another area attracting considerable attention and investment in the interventional radiology market is hemodialysis access devices. The demand for devices used in renal dialysis is expanding rapidly because of the growing prevalence of end-stage renal disease and the resulting increase in dialysis patients. As shown in Table 5, the number of hemodialysis patients increased at a 5.3% annual rate between 1995 and 1999 (year 2000 data is extrapolated). Correspondingly, the number of surgical procedures performed to install dialysis shunts also increased, as indicated by the data pertaining to the Medicare population over the 1995-2000 period. The more rapid growth in dialysis shunt procedures indicates that an increasing proportion of patients over 65 are undergoing hemodialysis, and perhaps a drop in the interval between shunt procedures for a given dialysis patient. The rapid growth in hemodialysis utilization is driving demands for products used in shunts, as well as for products used to improve shunt performance, including devices for maintaining patency, devices for restoring patency in thrombosed shunts, and hemodialysis catheters and related access devices. Pressures to reduce costs and improve patient convenience are also creating demands for higher flow rates during dialysis procedures, impacting catheter design requirements.
 |
The most vexing problem for hemodialysis patients is shunt failure, usually due to thrombosis at the outflow side. A major government initiative is helping to promote greater use of native fistulae as opposed to prosthetic grafts for dialysis shunts, since typical patency for an arterio-venous fistula is 45% to 55% at three years, whereas synthetic grafts exhibit a patency of about 40% at one year, according to Karim Valji, MD, of the University of California San Diego Medical Center (San Diego, California). However, the increasingly poor blood vessel quality in the dialysis population has limited the ability to use native dialysis fistulae to about 26% of hemodialysis patients. New technologies such as anti-restenosis treatments and coatings to improve hemocompatibility developed for coronary stents offer one potential solution that could improve patency of prosthetic grafts, but one that will take time to implement.
Considerable progress has been made in techniques to clear clotted shunts. One approach that is rapidly gaining acceptance is the "lyse-and-wait" technique, introduced by Cynanmon in 1997. The method employs a drug infusion catheter such as the UniFuse from AngioDynamics to infuse a thrombolytic agent such as tissue plasminogen activator into the clot while both ends of the shunt are compressed. Compression is maintained for between five minutes and one hour until the clot is dissolved, and the shunt is then cleared. Synthetic grafts are easier to clear than fistulae since the vascular anatomy can be more difficult to ascertain and aneurysms also can complicate matters, providing additional disincentive to use a dialysis fistula.
A number of devices have been developed to aid in clearing clots from dialysis shunts. The Helix Clot Buster manufactured by Microvena is an example. The company has introduced the Dual Wire Apex technique requiring only a single needle stick to facilitate access with the Helix in dialysis grafts. The Helix is priced at $550, or $900 per catheter for the 75 cm or 120 cm version, and does not require an expensive drive console. Another device, exhibited for the first time at the SCVIR conference, is the Resolution ultrasonic endovascular ablation system from OmniSonics Medical Technologies (Wilmington, Massachusetts). The initial target application for the Resolution System is thrombolysis of clotted dialysis access grafts. The system consists of an ultrasonic generator, a handpiece that converts the energy from the generator to a specially tuned probe, and a 0.020-inch diameter probe used to deliver the ultrasound energy to the clot. The technology works by inducing cavitation in the target material, preferentially attacking the most rigid structures. It ablates thrombus to a particulate size of approximately 10 microns. In vivo studies have not demonstrated any significant effect on blood vessel walls. The technique requires one minute at most for clot dissolution based on results of model in-vitro studies, considerably less than for thrombolysis, and eliminates the risk of hemorrhage associated with thrombolytic drug use. OmniSonics has initiated clinical studies in the U.S. and Europe. Other devices that have been used to clear clotted dialysis grafts include the AngioJet from Possis Medical (Minneapolis, Minnesota) and the Trerotola PTD catheter manufactured by Arrow International (Reading, Pennsylvania).
Another area attracting considerable interest is the use of stents and stent-grafts to improve patency in dialysis shunts. Typical sites for stent placement include the venous anastomosis, the venous outflow, or the central veins in proximity to the graft. The aSpire covered stent from Vascular Architects is one device that has been evaluated for treatment of hemodialysis graft stenosis. A study in 17 patients with dysfunctional PTFE hemodialysis grafts using the aSpire demonstrated a 100% success rate in stent placement, and a reduction in the diameter stenosis from 22% after angioplasty to 9% after stent placement. At 30 days, graft patency in the study population was 67%. Other stents that have been evaluated for treatment of stenotic grafts include the Boston Scientific Wallstent, the Cordis SMART stent and an ePTFE-covered stent from Impra/C.R. Bard. A study reported at the SCVIR sessions by P.M. Vogel, MD, of the Sutter Institute for Medical Research (Sacramento, California), found a major improvement in duration of patency for stents placed in the central veins to treat hemodialysis access venous stenosis with the Cordis S.M.A.R.T. stent. The median graft survival time was 16.3 months with the S.M.A.R.T. stent vs. 3.9 months with the Wallstent. For peripheral veins, the median graft survival time was 6.4 months with the S.M.A.R.T. stent vs. two months with the Wallstent. Vogel stated that stent movement at the leading or trailing edge of the stent may hamper Wallstent patency. A study of 21 patients receiving implants of the Impra ePTFE-covered stent showed 70% primary patency, with no intimal hyperplasia within the stent.
Other approaches to improving patency in dialysis access shunts include external beam radiation and intravascular brachytherapy to prevent tissue in-growth. Although external beam radiation is attractive because of its noninvasive nature, a small trial using that modality showed no improvement in patency. Novoste (Norcross, Georgia) recently submitted an IDE application to begin clinical studies with its Corona beta intravascular brachytherapy system to treat arterial-venous dialysis graft stenosis. The company will conduct the BRAVO (Beta Radiation for treatment of Arterial-Venous graft Outflow) trial to study the use of its Beta-Cath system in dialysis access grafts, which will be the first clinical evaluation of intravascular brachytherapy in that application.
Another major segment of the dialysis access products market is dialysis catheters and implantable ports used for both temporary and long-term dialysis. Temporary or short-term catheters are used in patients who require dialysis while waiting for their A-V fistula to mature or prior to prosthetic graft placement, and in other patients requiring only short-term dialysis. Long-term dialysis catheters are used as a last resort in patients whose fistula or graft fails and cannot be salvaged. At present, suppliers estimate that about 50% to 55% of dialysis patients have prosthetic grafts, 26% have fistulae, and the remaining 19% to 24% use catheters. An estimated 250,000 hemodialysis catheters are placed each year. Leading products include the Hickman silicone catheter, the OptiFlow catheter, and the new Hemo-Glide polyurethane long-term dialysis catheter from C.R. Bard; the Tesio-Cath, Ash Split, Magna, Hemo-Cath, Ultra-Flow and Hemo-Flow long-term catheters as well as a line of short-term catheters from MedComp (Harleysville, Pennsylvania) and the More-Flow long-term high-flow catheter from AngioDynamics. A new split hemodialysis catheter from Spire Biomedical (Bedford, Massachusetts) was cleared for marketing in mid-April. Key features required by users, according to Donald Denny, MD, of Princeton Radiology Associates (Princeton, New Jersey), include flow rates of greater than 400 ml per minute; no fibrin or thrombin adherence to the catheter lumen; lack of kinking; and a cuffed or tunneled design. Infections are a major issue with hemodialysis catheters, occurring in half of all patients according to Denny. In fact, infections are the cause of death for 14% of all dialysis patients. The use of higher flow rates is another important trend in hemodialysis.
An alternative is an implantable port system such as the LifeSite from Vasca (Tewksbury, Massachusetts). Vasca, a venture-backed firm that is considering an IPO in the near future, launched its device for use as a bridge to fistula in September 2000. Since then, more than 4,000 patients have used the device. The LifeSite provides a high flow rate of 400 ml to 500 ml per minute. A study reported at the SCVIR sessions by Melvin Rosenblatt, MD, of Memorial Sloan Kettering Cancer Center (New York), showed a 47% lower device-related infection rate and a 75% lower rate of thrombolysis as compared to the Tesio-Cath hemodialysis catheter.
