BB&T Contributing Editor
LONDON - The annual meeting of the Spinal Arthroplasty Society (SAS; Aurora, Illinois) convened at the ExCel Center in the revitalized Docklands area. The 1,500 attendees were treated to an intense program of workshops, 502 podium, oral and poster presentations and about 75 exhibiting companies.
The society was founded nine years ago to focus on motion preservation technologies as an alternative to spinal fusion. Stephen Hochschuler, MD, a founder and past president of SAS and chairman of the Texas Back Insitute (Plano, Texas), explained that the society's scope is changing and now includes minimally invasive surgery, biologics and innovative fusion technologies. He has authored texts titled "Back in Shape" and "Treat Your Back Without Surgery."
The market for spinal implants is crowded with a proliferation of large and small companies. Estimates of spinal devices on the market or under development include over 120 pedicle screw systems, more than 30 total disc replacements and 15 to 20 nucleus replacement products. Many of these products are being sold in Europe and other international markets but are not yet approved for sale in the U.S.
The market is poised for consolidation with some of the early stage companies with limited financial resources are at risk of disappearing from the scene. The prospect for completing an initial public offering in today's economy is dim and only three companies that market exclusively spinal products are publicly traded, namely, Trans1 (Wilmington, North Carolina), Alphatec Spine (Carlsbad, California) and NuVasive (San Diego)
Devices for motion preservation, dynamic stabilization
Facet Solutions (Hopkinton, Massachusetts) has completed a one-year follow up of a 30-patient pilot study on Acadia, its cobalt-chrome facet replacement device that utilizes conventional pedicle screw fixation. Enrollment is under way for a multi-center pivotal trial in the U.S.
Impliant (Ramat Poleg, Israel and Princeton) is conducting an IDE study on 300 to 450 patients at 21 sites in the U.S. on its TOPS system, a mobile pedicle screw-based device that is implanted posteriorly to reconstruct the motion segment. It is a unitary device composed of a titanium component with an interlocking polycarbonate urethane articulating core. It mimics the natural kinematics of the spine. The company had made a change in the device's initial design by modifying its articulating elements to reduce stress on the polymer component.
Spine Vision (Paris/San Francisco) exhibited FlexPLUS, a dynamic stabilization system. It provides lumbar spine restabilization after decompression in dynamic or static lumbar stenosis to prevent kyphotic imbalance. It is marketed worldwide, but not in the U.S. The company plans to submit a 510(k) to the FDA. UNI-Thread is a thoraco-lumbar spinal system for treating lumbo-sacral spine disorders and for reduction of spondylolisthesis. The company's new UNI-Thread SPL device permits unconstrained anterioposterior reduction of listhesis. These products are sold in the U.S. along with the Plus and X Plus pivot link universal systems.
Eden Spine (Lake Mary, Florida) exhibited its Wellex interspinous device, which was recently launched in Europe. It is seeking the CE mark and 510(k) approval for the FX-1 rod, a posterior dynamic stabilization device for treating severe stenosis. It controls movement dynamically in all planes and all directions.
Proliferation of new spinal Devices
The development of new spinal implant devices continues unabated. These include, pedicle screw systems, cervical and lumbar total disc replacements, interbody spacers and plating systems
Scient'x (Guyancourt, France/West Chester, Pennsylvania) received the CE mark for its Isobar TTL lumbar In System, an addition to its line of thoraco-lumbar pedicle screw based systems that is implanted via a posterior approach. Its top-loading inner locking nut provides strong axial resistance and reduces the screw head profile It is fully compatible with the company's Isobar dynamic rod. Discocerv cervical disc prosthesis is the first ceramic cervical disc. It is implanted between C3 and C7 via the anterior route.
Globus Medical (Audubon, Pennsylvania) has introduced several new devices. Coalition is a stand alone plate-spacer combination. It is a one-piece cervical insertion device comprised of two components and is inserted flush with the vertebrae. Independent is a similar device that is used for anterior lumbar interbody fusion.
The Transition dynamic stabilization system can be preassembled or assembled in the operating room. It competes against the Dynesis device from Zimmer Spine (Edina, Minnesota), a subsidiary of Zimmer (Warsaw, Indiana), that requires in situ assembly during surgery.
The Revolve posterior minimally invasive stabilization system is the next generation of the company's Pivot pedicle screw system. It uses a pedicle screw with a narrow rod. It has multi-level capabilities and can do 6 or 7 levels, whereas, Pivot can do only 1 or 2 levels.
Disc Motion (Boca Raton, Florida) markets InLign TMS (total motion segment), a posterior lumbar spinal arthroplasty system, InLign MAS (multi-axial screw) that can be used with a rigid rod or with the InLign ARC (anatomic radius component) as an adjunct to fusion. The multi-axial screw reduces torque on the bone-screw interface to minimize screw loosening. These devices are sold in Europe and Turkey but not in the U.S.
The company is in the middle of 510(k) submissions for the InLign MAS and InLign ARC devices. Clinical trials are under way on InLign TMS with 27 of 30 cases completed without any reported complications. Disc Motion plans to file with the FDA for an IDE study.
Aesculap Implant Systems (Central Valley, Pennsylvania), a division of the B.Braun Group (Tuttlingen, Germany), launched its RS4 posterior cervical system in the U.S. and Europe. It is the smallest and strongest commercial product of this type. It is conducting an IDE trial on its Actv L lumbar disc which it sells in Europe where it also sells the Actv C cervical disc.
NuVasive has initiated a 300-patient clinical trial at 15-20 sites on a lumbar total disc replacement device that is inserted laterally at the L 4,5 and above. It builds on the company's MAS Xlif platform for thoraco-lumbar surgery which uses a lateral XLIF approach along with NeuroVision intraoperative monitoring, a nerve and vessel avoidance system.
NeoDisc, an investigational device in the U.S., replaces a natural intervertebral cervical disc that has degenerated. It is designed to replicate the cushioning and motion characteristics of an intact healthy cervical disc and minimize stress to the surrounding spinal structures.
NuVasive announced in its acquisition of Cervitech (Rockaway, New Jersey) for $47 million plus an additional $33 million upon FDA approval of Cervitech's PCM cervical disc designed as a full motion implant which is currently in a 400-patient clinical trial at 24 sites in the U.S. The trial is expected to be completed before the end of this year and a PMA will be submitted early next year.
Nexgen Spine (Whippany, New Jersey) has developed a proprietary approach for attaching a polycarbonate polyurethane elastomer to a metallic endplate without using a chemical adhesive. It has begun distribution in Europe of its Physio-L artificial intervertebral lumbar disc implant indicated for use between L3 and S1. It is comprised titanium endplates above and below a central elastomeric core.
An IDE will be pursued later this year. The Physio-C artificial cervical disc is indicated for use between C3 and C7 and the CE Mark is expected to be obtained early in 2010.
X-Spine Systems (Miamisburg, Ohio) is developing laser shock processing technology for producing stronger titanium implants with high fatigue life. It currently markets the Spider cervical plating system and the Calix cervical interbody spacer. Its thoracolumbar products include the Capless pedicle screw system with rotary locking technology and the Butrex anterior lumbar plating system. Hydragraft is a hydratable human allograft that is specifically designed for spinal applications.
Orthofix Spine/Blackstone Medical (Wayne, New Jersey) plans to launch the following three new products in the U.S. and in Europe by the end of the year: Firebird pedicle screw system which is an improvement over its ICON pedicle screw and is available preassembled or modular design; Pillar PEEK spacer system, a stand alone anterior interbody device that has four screws located medially; and Ascent, a posterior occipital cervico-thoracic system.
Zyga Technology (Minneapolis) is a venture-backed company that is developing a device for resurfacing articular cartilage and facet joints. The company plans to initial clinical trials before the end of this year.
Devices for minimally invasive spinal surgery
Alphatec Spine (Carlsbad, California) sells in Europe the OsseoFix spinal fracture reduction system for the stabilization and reduction of spinal fractures by providing internal fixation using a titanium implant in conjunction with polymethylmethacrylate.
The company is awaiting a response from the FDA on its 510(k) filing for this device. Its Illico MIS retractor system with percutaneous pedicle screw represents the company's entry platform in minimally invasive surgical procedures. It is used for posterior fixation of disorders in the thoracic and lumbar spine. The product is sold in the U.S. and Europe and has been submitted for approval to market in Japan.
Vexim (Balma, France) has conducted clinical trials on over 100 patients for SpineJack. It is used by a minimally invasive procedure to treat vertebral compression fractures and restore height, endplate anatomy and corner profile. The company plans to launch the product in Europe this year and to submit a 510 (k) to the FDA by the end of this year.
Non-Linear Technologies (Or Yehuda, Israel) is developing the Prow Suite spinal implants and tools that offer motion preservation and a percutaneous alternative to surgery for treating degenerative disc diseases. The company's first target is for treating lumbar spinal canal stenosis.
Its patented technology enables a novel (non-linear) minimally invasive technique for introducing instruments and implants into the body. The Prow Suite is composed of three independent units, posterior and anterior implants and a discectomy tool that may be used separately or combined.
Products for nucleus replacement
Disc Dynamics (Eden Prairie, Minnesota) sells its DASCOR disc arthroplasty system in the UK, Germany and Belgium. It consists of a two-part curable polyurethane and an expandable polyurethane balloon which is inserted in the disc nucleus space after the nucleus has been removed. The balloon is then injected with the flowable polymer to create an implant that conforms to the shape and size of the disc space. The procedure is performed in a few minutes.
The company has almost completed a 40-patient pilot study and will enter a pivotal IDE clinical trial in the U.S. on 300 to 500 patients.
Pioneer Surgical Technology's (Marquette, Wisconsin) NuBac is the only other disc arthroplasty device sold in Europe.
Raymedica (Minneapolis) was the leading company in nucleus arthroplasty with its PDN-SOLO device, but it was discontinued due to its poor performance. The company has merged with Surgicraft Group Holdings (Reddich, UK) and been renamed Centinel Spine (Minneapolis).
The two devices that it markets in the U.S., Europe and Australia are Stalif TT and Stalif C, stand-alone lumbar and cervical interbody fusion devices made of radiolucent PEEK Optima.
Replication Medical (Cranbury, New Jersey) is developing spinal products based on H-PAN, a proprietary polyacrylonitrile-based hydrogel. Its NeuDisc is a nucleus replacement that has been redesigned to increase stiffness and durability and is in clinical trials in China. The EnGuard vessel guard is composed of Dacron mesh impregnated with hydrogel and recently received 510(k) approval for anterior use in spinal surgery. It is ready for marketing.
The company also is developing an interspinous spacer that uses the same hydrogel. It is similar to the X-Stop metal spacer from Medtronic Spine & Biologics (Memphis), a subsidiary of Medtronic (Minneapolis), but is smaller and expands after implantation.
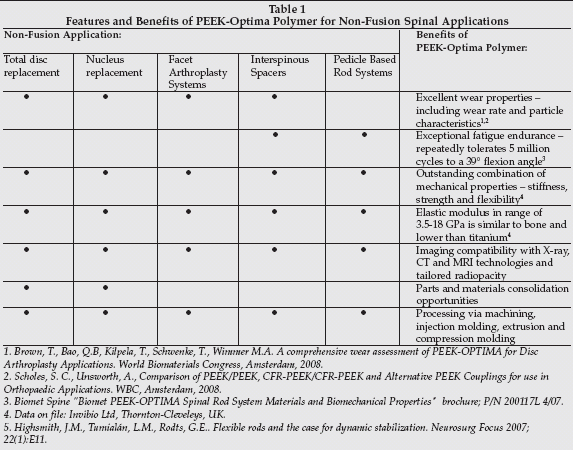
PEEK use expanding in spinal implants
PEEK (polyetheretherketone) is at the forefront in the biomaterials field as a radiolucent replacement for metals in spinal implants due to its high strength and wear properties and modulus of elasticity is close to bone. Metal devices can obscure soft and hard tissue integration to implants during evaluation by X-ray or MRI. PEEK has supplanted titanium in interbody spinal fusion cages.
Invibio (West Conshohocken, Pennsylvania), a subsidiary of Victrex (Lancashire, UK) is the global market leader for implantable PEEK-based biomaterials. PEEK Optima polymer has found widespread use in medical devices. Applications of this polymer have gone beyond spinal fusion cages and is being used in pedicle based stabilization rods, interspinous spacers and in nucleus replacement devices (see Table 1).
 |
Signus Medizintechnik (Alzenau, Germany) and Signus Medical (Chanhassen, Minnesota) are sister companies that market cervical and lumbar interspinous spacers, pedicle screws, cervical plates, and the most complete line of PEEK Optima implants in the spinal market. Galileo is a new cervical disc prosthesis with controlled translation that is independent of rotation. It is implanted as a single component like a cage and is sold in Europe but not in the U.S.
Pioneer Surgical Technology reviewed its 300-patient U.S. study for implanting its NuBac disc arthroplasty device by the posterior approach as well as by the more established lateral and anteriolateral approaches. The company has a CE Mark for the posterior approach and has started an IDE study in the U.S.
NuBac uses a PEEK-on-PEEK design which has better wear characteristics compared to polyurethane or metal-on-metal. The company is conducting clinical trials on this device in the U.S.
Its BacJac interspinous spacer is sold in Europe where it is gaining market share from the X-Stop spacer because it is easier to use. NuNec is Pioneer's next generation disc arthroplasty device and plans an IDE study on this product.
Novel biomaterials for spine surgery
Doctors Research Group (Southbury, Connecticut) markets in Europe and Canada its Kryptonite osteoconductive adhesive with bone-like properties. It is composed of naturally occurring fatty acids (caster oil) and calcium carbonate. The product is prepared by mixing a pre-polymer, a polyol and calcium carbonate. The company plans to soon submit for FDA market clearance.
K2M (Leesburg, Virginia) showcased an injectable polymer for which it acquired worldwide rights from Promethean Surgical Devices (Woburn, Massachusetts) for use in all spinal applications. It is a porous polyurethane that covalently binds to soft tissue Promethean had previously tried unsuccessfully to use the polymer as a bulking agent to treat incontinence and is currently evaluating it as a coating on mesh for hernia repair.
The polymer will serve as a platform for the development of new implantable products such as a spinal nucleus replacement and micro-access surgical approaches for annular decompression and repair. K2M's primary business is as a marketer of products to treat scoliosis. Its Serengeti screw-based retractor system is a breakthrough in minimally invasive spinal surgery.
ApaTech (Elstree, UK) produces Actifuse ABX, a moldable, silicate substituted osteostimulating bone graft. The presence of silicate in calcium phosphate increases the surface negative charge thereby attracting more cell binding proteins immediately after implantation. It is available in granular, putty and shaped forms.
Actifuse MIS is a device used to deliver the synthetic bone graft. The products are sold in 21 countries, including the U.S., Australia and most countries in Europe.
A-Spine Holding Group (Taipei, Taiwan) sells Osteo-G, a bone void filler comprised of a strontium-substituted hydroxyapatite. It featured Vessel-X, which it sells in Europe and Asia and plans for future marketing in the U.S. It is a non-stretchable fabric that is serves as a bone filling container and is used to restore the height of a vertebral body and reduce risk of cement leakage in the vertebral body. It is described as a new kyphoplasty product since no balloon is required.
