BB&T
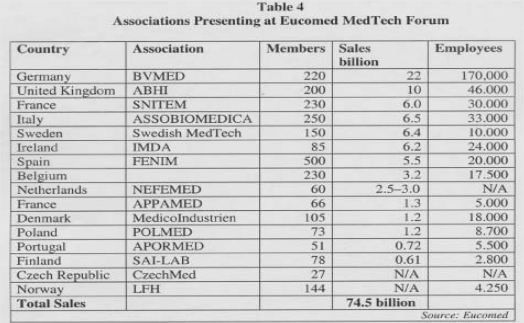
BRUSSELS, Belgium – As a show of strength and solidarity for an emerging agenda of activism on the European scene, Eucomed (Brussels) brought together its stakeholders on the second day of its first-ever MedTech Forum here in mid-October. A total of 16 national associations (see Table 4) set up camp in an exhibition area while many leaders of the largest medical device manufacturers held forth in panel sessions.
 |
The centerpiece for the discussion were findings from a specially commissioned survey by McKinsey & Co. (London) and report leader Martin Dewhurst offered a review of key trends and challenges for the industry that packed the plenary session. While the study sounds low-powered at 80 respondents, the participation was high-level with 50 being CEOs of European operations for multinationals and the remainder being heads of regional operations. The report showed executives upbeat and aligned in agreement on many key points regarding business prospects.
Some key findings:
• Demand for existing products will continue to increase according to 93% of those surveyed.
• The statement that there are "pockets of attractive growth opportunities" in developed markets of Europe won 86% agreement.
• Untapped opportunities include the shift in care settings for patients and the wild variations in clinical practice across Europe, such as the fact Germany has 80% more hip replacements than Italy while there is no clinical reason for this variation.
• European executives also share a conviction that there are significant unmet needs for medical devices in their developed markets that will drive innovation. More than 90% of respondents said there were "exciting drivers for growth in the emerging markets of Eastern Europe, the Middle East and Africa."
• Top issues identified for Europe were regulation, reimbursement requirements and tendering processes.
• More than 80% of the executives share the belief that generics pose a credible threat to their business, and several affirmed a prediction by McKinsey's Dewhurst that between 30% and 40% of their current product portfolios are vulnerable to "commoditization."
Dewhurst said that despite the enthusiasm among med-tech executives for emerging opportunities in China, India and Brazil, the region defined as Europe, the Middle East and Africa "represents the biggest market bloc and will remain so over the next 20 years."
Turbulence ahead in the 'Healthcare Century'
The challenges cited as being faced by the industry identified came as no surprise, and are aligned with the Eucomed agenda, for which the trade association has been building industry support.
In his presentation of trends for what he termed "The Healthcare Century," Dewhurst showed that the spending in the sector, which has outstripped gross domestic product (GDP) by 2% every year for the past 50 years shows no signs of slowing and a train wreck is coming.
"If the growth was slowed by half to just 1% each year above GDP, which no government has been able to do," he said, healthcare spending in the U,S. will grow to 40% of GDP.
The aging of the population in Europe means that maintaining the current benefit levels for citizens will require in 2030 a 140% increase in taxes in Italy, 90% in Germany and 70% in France.
"A turbulent period is coming for the industry with a risk of misplaced or disruptive healthcare reforms" where governments desperately slash services and cut prices in an effort to cope with the runaway trends.
"There is a huge imperative for everyone, and importantly for industry, to become engaged in the solutions to these problems of access, quality, productivity and affordability in order to shape the future," he said.
A panelist from the first day of the MedTech Forum, Sabine LeCrenier, the head of the unit responsible for medical device regulation with the European Commission's (EC) Directorate General (DG) Enterprises and Industries, made a repeat appearance on the second day, taking to the podium for a presentation on the European Union's Agenda for Medical Devices.
Everyone seemed to be holding their breath as she worked through praises for the industry's role in the European economy, like waiting for someone to complete the phrase, "What a nice person, but ..."
What came next left industry regulatory experts surprised, somewhat pleased and, as always, cautious.
"It was not what she said that was important, but what she did not say," said Hauke Schik, quality and regulation manager with Philips Healthcare (Eindehoven, the Netherlands).
"We did not hear anything about the European Medications Agency (EMEA), and that was a surprise," he said, referring to the EC's proposal this summer to recast the Medical Device Directives by grouping medical device regulatory authority under the aegis of that agency.
"It was very surprising," agreed Dario Pirovano, director for medical device legislation at Eucomed, who wryly suggested LeCrenier was being diplomatic."Having seen the reaction of industry to this proposal, and especially the very mention of EMEA as the authority, she may have been reluctant to mention it again in a closed room full of industry executives," he said.
A second surprise was an overt statement that the "New Approach" regulatory method used in Europe since 1993, where industry is responsible to police itself across several broad categories of devices, will remain in place.
Instead the commission seems to have shifted its position toward carving out the emerging category of biodevices that have raised concerns over what she called "gaps and loopholes" in regulations.
Pirovano parsed the commission's position for BB&T, saying, "She did not refer expressly to innovative devices as being part of the technologies they are concerned need a higher scrutiny. She said some category of devices may need this scrutiny but that 'most' medical devices will remain under the New Approach. She did not say 'all' so this needs to be clarified."
"The real bombshell in her presentation was to hear that last week the commission created a group to study reimbursement and pricing of medical devices across Europe," he said.
"My first reaction was quite positive," he said, adding that since lunch he had become cautious, waiting to hear more specifics, "yet it is very encouraging they are going to address this topic."
Shrik said it is clear the commission "has understood the message about reimbursement issues in Europe, that there can not be innovative medical systems without a means to pay for it, so this is good."
Med-tech firms outline their concerns
In the middle of a morning filled with Brussels-speak, the European version of the same language spoken by policymakers in Washington, there suddenly came a clear voice that boomed over the microphone with a distinctly Midwestern U.S. accent.
"What the hell can you do to help us?" asked Luciano Cattani, executive vice president-international public affairs for Stryker (Kalamazoo, Michigan), who also is the vice chairman of Eucomed, which organized the "Medical Device Value Day" session as part of the MedTech Forum.
Perhaps Cattani chose to be so blunt because it was a rare opportunity to put the question to two highly influential policymakers for medical devices, Ludovica Serafini, a European Commissioner responsible for the Directorate General (DG) for Research, and LeCrenier.
"We need help," Cattani said. "We have late payments, procurement price registries in Italy and soon in the Netherlands, and we are getting closer to a pharmaceutical model of regulations. Everywhere we see a tightening and a severity toward medical devices and frankly, we do not see a light at the end of the tunnel."
The response from the representatives of the EC was polite and necessarily indirect, as the EC has only soft tools available to influence the implementation of its recommended rules among the 27 member states.
LeCrenier said the role of industry is to provide information to the EC to help it achieve "a balanced approach," Brussels terminology for "reflection" of input from every possible interest group before issuing any statement, ruling or regulation.
"We need cooperation because we can not know all your programs and products," she said, adding pointedly, "work with us in a constructive way."
Herb Riband, vice president for legal and external affairs for Medtronic International Trading (Tolochenaz, Switzerland), tried a different approach, saying "Thank you for taking up procurement and pricing. We need some best practices identified in this area, as they can impose barriers to access to medical technologies."
The exchange between the highest-placed authorities of Europe and representatives of companies with the highest market share for medical devices sold in Europe was the highlight of a session where Eucomed sought to bolster an evidence-based argument favoring a separate consideration of regulation for medical technology with the EC that suddenly this summer proposed grouping devices with drugs under the control of a single agency.
"We are having a better dialogue with the Commission on this issue," Cattini told MDD following the session, when he had rediscovered his Brussels voice.
"We are presenting evidence now to start moving devices out of being considered as a sub-class of drugs and to build a greater appreciation of how much the industry contributes to gross domestic product, and especially to job creation," he said.
Value proposition for med-tech
In a series of presentations, Eucomed rolled out the fruit of the labors of the European Health Technology Institute on Socio-Economic Research (EHTI) that it created in 2007 to provide robust information on the social and economic value of medical technology.
Three prestigious universities collaborated to provide the first-ever comparison of how regulation and reimbursement affect six different devices in a "health basket" across the five major markets of Western Europe.
The products selected for the comparative study were grouped in three categories: Medical Aids, with incontinence pads and negative pressure wound care therapy; Implants, with cardiovascular defibrillators, knee endoprostheses and coronary stents; and Assistance for Professionals, with laparoscopic colorectal surgical devices.
Reimbursement status for each device and any incentives are detailed with data.
The study of "Financing Medical Devices in Germany" was led by Professor Reinhard Busse, head of the department for healthcare management at Technische Universität (Berlin), who explained in his report, "Although the European Union (EU) directives may give the impression of uniform regulation in the EU, all regulations concerning the financing of medical devices are made at the level of the member states.
"Thus, there are in principal 27 different national approaches regarding the financing of medical devices," he wrote, adding, "in some countries decisions concerning the financing of medical devices are taken at a regional level."
Touching on the heart of Eucomed's purpose in funding the research, Busse said, "There is broad evidence that improvements in life expectancy may be associated with innovative medical devices. However, up to now, the number of analyses dealing with the financing of medical devices is rather limited, compared with studies analyzing the policies dealing with pharmaceuticals.
An identically structured report detailing the financing of medical devices in France and the UK was led by Professor Panos Kanavos with the department of social policy at the London School of Economics.
The report on Italy and Spain was led by Professor Elio Borgonovi with the Center for Research on Health and Social Care Management at the Università Bocconi (Milan, Italy).
The shared experience for marketing a medical device in Europe is that while it seems easier to get to market with the CE mark, things get decidedly tougher the closer you come to local buyers.
The conclusions in the reports from the three research teams, which are made up of in-country experts intimate with the health systems under study confirm the complexity with labyrinthine layers of rules, pricing and reimbursement that varies between regions and even local governments.
In Germany, which is presented as having a more up-to-date and coherent system, Busse warned that manufacturers should stop complaining about reference pricing points because there is a greater menace on the horizon with a trend toward public tendering. where price points so far are coming in 30% lower still.
France and the UK share a national authority over health policy, but both have been steadily decentralizing their systems — France towards its hospitals and the UK towards local cares organizations, called trusts.
Purchasing follows the pattern, with regional groups formed around hospital centers in France and a patchwork decentralization in the UK, where the bulk of procurement is done regionally or locally through trusts, primary care trusts and emerging collaborative procurement hubs.
Procurement in both countries is generally by public tendering with price being the nearly unique hot point in France, while there is a greater emphasis, in principal, the authors note, on value-for-money in the UK with evidence-based procurement.
There is a move toward centralization in Italy and Spain to address the deeply fragmented landscapes formed by political divisions that stretch back to the dawn of healthcare.
Yet centralization in these countries has nothing to do with a northern European's ideas and it is being done at different levels and with different mechanisms, the Boccioni team notes.
Compounding the problem of knowing who is going to pay is the obsolescence in both countries of the reimbursement rates for technologies and devices.
In Spain, the maximum prices to be paid for what are now outdated technologies were set in 1988 and have not been revisited.
In Italy, national caps on prices were removed in 1999, with current ceilings based exclusively on negotiated quantities.
The authors warn that a major issue for device providers is that they need to be prepared to pay out-of-pocket for services necessary to support a new piece of hardware, "since in both countries funding systems cannot automatically guarantee that additional resources are made available to new health technologies."
Wrapping up the "Value Day" discussions, Eucomed issued a Brussels-speak press release that aimed to point the way forward saying its next steps are to "further engage with the Commission in a constructive dialogue to address the identified concerns with solutions that are supportive of innovation and patient safety."
Meanwhile, at the assembly of its members Eucomed is winning financial support for a new round of research to document procurement and late-payment practices across the continent.
More bandwidth for industry's voice
The voice of the medical technology industry in Europe needs more bandwidth, according to John Wilkinson, the new director general of Eucomed. The prospect of tighter regulation from the European Union (EU) seems increasingly likely, and even worse in the eyes of seven affected industry associations, it will probably be rolled into the authority of a superagency covering both medications and devices.
Called a recast of the Medical Device Directives, the legislation proposed for debate next year would end a 23-year history of industry self-regulation and, according to opponents, will effectively kill the one advantage Europe has held in international competition, which is speed to market.
Meanwhile manufacturers are feeling more sharply than ever the deliberately slow payment of public health authorities, the primary payers for healthcare in Europe, which in some countries have all but formally adopted the practice to camouflage ever-deepening expenses.
Finally, there is a full agenda of issues now being defined under the banner of patient safety that would have significant impact on markets from single-use devices to health information systems.
The three-day MedTech event was the trade group's first full-scale external event gathering dozens of national associations to meet with their delegations at the European Union and winning the support from several multinational heavyweights such as Johnson & Johnson (New Brunswick, New Jersey) and Medtronic (Minneapolis).
More than 400 participants took part in the three session tracks focused on regulation, patient safety and economic value.
"I rather like bandwidth as a way of describing this," said Wilkinson, who formerly was the head of the Association of British Healthcare Industries in the UK, serving four years as the director general.
"People are not just turning up here, they are engaging this week with representatives' offices from their nations," he said. "However effective we are as an organization, we will never be as effective as these organizations all working with the policymakers, so I call this increasing the bandwidth for Eucomed."
Traditionally an internally focused association, he explained, the MedTech Forum was built on top of the annual Eucomed General Assembly to force a shift toward external relations.
He cited the EHTI reports as a key step. Eucomed is a partner in EHTI with three of Europe's leading business schools – the London School of Economics, the Università Bocconi in Milan and the Technische Universität Berlin.
Wilkinson brings to Eucomed a deep experience pushing and prodding British policymakers that served as a warm-up for playing in the championship league in Brussels. He was influential in the creation of the Healthcare Industries Task Force (HITF) within the UK Department of Health and served as its co-chair. He was also a familiar face before the UK Health Select Committee, giving evidence and testifying on the cost benefits of new medical technologies and on the barriers to the adoption of innovations within the National Health Service (NHS).
In an interview with MDD, Wilkinson said, "Procurement is increasingly an issue for industry across Europe as there is a disconnect between professional being brought in to trim costs and the clinicians charged with treatment and workflows. The guiding principle for a procurement professional is to purchase whatever was purchased the previous year, but to buy it more cheaply."
"This is particularly a problem when you consider that new technologies being introduced often offer opportunities to restructure the way that we deliver healthcare, such that if you just carry on buying year after year but more cheaply, you devices from the needs of clinicians and miss a tremendous number of opportunities," he said.
