A CDU
It's a rather famous fact that patients frequently don't take the drugs they're prescribed and don't even have them filled at the pharmacy. This non-compliant behavior is intensified as drug regimens change and become more complicated, even with those drugs prescribed as must-takes to prevent severe heart problems, even death.
This is the basis for developing a simplified method for prescribing heart medication, Ross Feldman, MD, Gunton Professor of Therapeutics at the University of Western Ontario (London, Canada), told attendees at this year's scientific sessions of the American Heart Association (Dallas) in Orlando. And he went on to describe a simplified system, which he described as an algorithm, that improved patient compliance with their blood pressure medications.
The Simplified Treatment Intervention to Control Hypertension (STITCH) trial studied 2,104 patients with hypertension at 45 family practices in southwestern Ontario, Canada. In order to increase the number of people with hypertension who reduce their BP to "goal" levels, researchers wanted to see if there was a simpler way to direct treatment for hypertension than by following national guidelines for optimal management of BP.
"The complexity of existing guidelines for the management of hypertension could be a barrier to effective therapy," said Feldman. He said that the study process involved a "cluster randomization trial," with "family practices randomly assigned to implement a simplified step-care algorithm (STITCH-care) or Guidelines-based care for the management of hypertension."
STITCH consists of four steps (see Table 4).
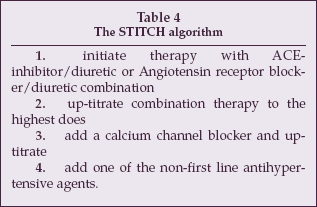 |
In the Guidelines-care arm, physicians were educated on the use of existing national guidelines of the Canadian Hypertension Education Program, which list 12 options for initial therapy depending on the type of hypertension and co-existing medical conditions.
The proportion of patients who reduced BP to the target level was higher in the STITCH-care group (65%) compared with the Guidelines-care group (53%).
Average systolic BP (the top number in a BP measurement) was reduced by 23 mmHg in the STITCH-care arm, compared to a lesser reduction of 18 mmHg in the Guidelines-care arm. In addition, diastolic blood pressure (the bottom number) was reduced by 10 mmHg in the STITCH-care patients vs. 8 mmHg in the Guidelines-care group.
STITCH served to increase the chance of reaching optimal BP by more than 20%, Feldman reported. He called the protocol "implementable," acceptable by family physicians and resulting in improved BP, due to the better compliance.
"This return to 'step-care' may be an important way forward for the treatment of hypertension and may be a paradigm for the management of a range of chronic diseases."
Beta blockers — mixed results in non-cardiac surgeries
Beta blockers might help protect patients who are at risk for atherosclerotic cardiovascular disease from suffering post-operative cardiovascular events, such as heart attacks, when they undergo non-cardiac surgery, according to another research report presented at the AHA scientific sessions. The Perioperative Ischemic Evaluation (POISE) trial is the largest, randomized controlled trial to access whether risks of post-operative cardiovascular events can be lowered by beta blockers.
The study enrolled 8,351 patients to determine the impact of the beta blocker metoprolol on the 30-day risk of major cardiovascular events in people undergoing non-cardiac surgery and at risk of atherosclerotic cardiovascular disease.
"Our study found strong evidence that perioperative CR metoprolol prevents heart attacks, but there is also concerning evidence that it increases the risk of death and stroke," said P.J. Devereaux, MD, principal trial investigator and assistant professor in the department of clinical epidemiology and biostatistics at McMaster University (Hamilton, Ontario).
"Findings also demonstrated that with metoprolol there was a decrease in atrial fibrillation and in the need for coronary revascularization an increase in clinically significant hypotension and bradycardia."
Patients received an oral dose of 100 mg controlled-release (CR) metoprolol or placebo two to four hours before surgery and between zero and six hours after surgery. Twelve hours following the first post-operative dose, patients started taking daily doses of metoprolol or placebo at 200 mg and continued for 30 days after surgery. If at any time patients could not take doses orally, 15 mg of the drug or normal saline as a placebo was administered intravenously every six hours until patients were ready to switch back to oral doses.
HemaQuest's journey starts with A round $20 million
Backed by a decade of research in hemoglobin disorders, start-up firm HemaQuest Pharmaceuticals (Newton, Massachusetts), though only recently established, has hit the ground running, with $20 million in Series A funding and plans for filing an investigational new drug application on its first compound by the end of the year.
The company says it is aiming to develop drugs to treat hemoglobin diseases, starting with sickle cell anemia and beta thalassemia, inherited disorders with few treatment options.
HemaQuest's technology stems from a10-year collaboration between Susan Perrine and Douglas Faller, from Boston University, and George Stamatoyannoupolous and his wife, Thalia Papayannopoulou, from the University of Washington (Seattle). That work centered on mechanisms to stimulate hemoglobin production and ultimately led to the discovery of "whole family of molecules that were active in this regard," said Ronald Berenson, president/CEO of HemaQuest.
Those molecules have shown favorable pharmacokinetics, are orally available and, perhaps most important, have demonstrated a good safety profile, "which has been one of the major limitations" of existing therapies, especially for patients requiring chronic treatment, Berenson said. He said he contacted Stamatoyannoupolous about a year ago, because interested in taking those discoveries commercial.
HemaQuest said it anticipates submitting an IND this quarter for a lead candidate and might begin looking at other pipeline opportunities for broader types of anemia, neutropenia and other hematological disorders.
Asked whether the company has aspirations to create its own commercial and sales staff in the future, Berenson said, "the jury is still out." Since indications such as sickle cell anemia is a "fairly focused market," it might be possible for a small company to reach it with a concentrated sales force. But, he added, "the strength of the company lies" in its R&D expertise in blood-related disorders.
Synvista reports 1st dosing of alagebrium in 100-patient trial
Synvista Therapeutics (Montvale, New Jersey) reported dosing of the first patient in BENEFICIAL, a 100-patient Phase II clinical trial of alagebrium (ALT-711), a compound designed to act as an Advanced Glycation End-product (AGE)-cross-link breaker, in patients with chronic heart failure (CHF). The BENEFICIAL study, a placebo-controlled, randomized trial evaluating the efficacy and safety of alagebrium, is designed to measure the effect of alagebrium on exercise tolerance in patients with CHF.
Synvista said it plans to use the experience and results of this study to guide development of its Phase III program for alagebrium in CHF. The single-site Phase II trial is being conducted at the University Medical Centre (Groningen, The Netherlands); principal investigator is Adriaan Voors, MD, associate professor of cardiology.
The objective of the BENEFICIAL study is to improve maximal oxygen consumption (VO2max), highly correlated with an improvement in myocardial function in patients with CHF. Diastolic and systolic function will be measured by tissue velocity imaging with echocardiography.
"Alagebrium is the first orally active AGE-cross-link breaker that has been evaluated in preclinical and clinical studies, and studies using animal models of diabetes and aging have demonstrated its effect in reversing cardiac stiffening," said Voors. "We are encouraged by the potential that alagebrium has demonstrated in our preclinical models, and are aggressively pursuing clinical development in this indication."
Alagebrium has been studied in two human heart failure trials: the DIAMOND trial in patients with diastolic heart failure; and the PEDESTAL trial in patients with systolic heart failure. While both trials provided encouraging results, each had an open-label design and thus neither was able to draw definitive conclusions about the effectiveness of alagebrium in heart failure
Synvista also reported results of a series of preclinical studies revealing that a common blood protein, haptoglobin, binds to the core of high-density lipoprotein and that a defective haptoglobin variant (Hp2-2), founjd in 40% of the population, may induce dysfunction in HDL.
Further, the studies reported that exposure to Vitamin E can restore HDL functionally and the process of reverse cholesterol transport.
Palatin begins dosing in Phase I trial of CHF drug
Palatin Technologies (Cranbury, New Jersey) reported the initiation of dosing in a Phase 1 clinical trial of PL-3994, a long-acting analog of atrial natriuretic peptide (ANP), under development for treatment of acute decompensated congestive heart failure (CHF). Future plans include the development of PL-3994 for long-term administration in patients with chronic CHF.
The Phase 1 trial of PL-3994 is a double-blind, placebo-controlled, single-ascending dose study in healthy volunteers who will receive the drug subcutaneously.
The evaluations will include safety, tolerability, pharmacokinetics and several pharmacodynamic endpoints, including endogenous messenger levels such as cyclic guanosine monophosphate.
Dr. Trevor Hallam, executive VP for R&D for Palatin, said that PL-3994 is "a compound developed by Palatin scientists that has the potential to become a new tool for physicians to advance the treatment of congestive heart failure."
ANP is a naturally produced peptide hormone involved in regulation of water and sodium levels in the circulation. Palatin's PL-3994 is a peptidomimetic compound, which incorporates features of both natural ANP and small molecules.
The company said that preclinical studies have demonstrated that PL-3994 is highly potent, with a long duration of action related to diminished affinity for the natriuretic peptide clearance receptor and increased resistance to the enzyme neutral endopeptidase, both pathways for clearance of endogenous natriuretic peptides.
Archemix launches Phase IIa trial of anti-thrombotic
Archemix (Cambridge, Massachusetts), a biotech company developing aptamer therapeutics, said it has begun a Phase IIa clinical trial of ARC1779, which is designed to evaluate the safety and efficacy of ARC1779 as an anti-thrombotic in patients suffering from Acute Coronary Syndrome, or ACS, undergoing emergency percutaneous coronary intervention, or PCI. ARC1779 is an aptamer designed to bind to and inhibit the function of the protein known as von Willebrand Factor, or vWF. vWF initiates and promotes platelet clot formation in the arterial circulation of patients with ACS.
The multi-national trial is expected to enroll about 300 patients. The trial is a dose-ranging, randomized, double-blind study using ReoPro as a comparator. The co-primary efficacy endpoints of the trial will be the degree of myocardial perfusion and the deficit of blood supply to the working heart muscle, or myocardial ischemia, after PCI. The primary safety endpoint is bleeding, which will be measured by clinical observation.
Archemix also plans to use biomarkers to measure the extent of heart damage suffered by patients as well as the activation of the clotting system.
The Phase 1 trial was conducted in 47 healthy volunteers and was completed in March 2007. ARC1779 demonstrated dose- and concentration-dependent inhibition of plasma vWF activity and platelet function. No serious adverse events were reported in the trial and no subject was withdrawn from the trial due to an adverse event.
Aptamers are synthetically-derived oligonucleotides that bind to protein targets with high affinity and specificity and can be designed to have a specified duration of action.
Momenta shares fall as FDA turns down Heparin ANDA
Shares of Momenta Pharmaceuticals (Cambridge, Massachusetts) fell sharply earlier this month after the FDA rejected an abbreviated new drug application for M-Enoxaparin, a generic version of the Sanofi-Aventis's blood thinner Lovenox from Sanofi-Aventis (Bridgewater, New Jersey).
The agency informed Momenta and collaborator Sandoz, the generics division of Novartis (Bael, Switzerland), that it failed to adequately address M-Enoxaparin's potential for immunogenicity.
The companies said they are working to determine what additional information might be required, though Momenta executives said they believe that those issues can be resolved based on "detailed characterization of enoxaparin and on current medical and scientific literature."
Momenta and Sandoz filed the ANDA in August 2005 for M-Enoxaparin, a version of low-molecular-weight heparin for treating deep-vein thrombosis and cardiovascular conditions. The two companies have been working together on the product since 2003, and later expanded their deal to include development and commercialization in the European Union as well.
Away from its generics pipeline, Momenta is developing a next-generation heparin product for acute coronary syndromes. Last month, it started a Phase II study of M118, an intravenous anticoagulant, in patients with stable coronary artery disease who are undergoing a planned percutaneous coronary intervention.
