BB&T Contributing Editor
In the first part of our review of the uses of collagen ("The ultimate in biomaterials — common collagen, Biomedical Business & Technology, November 2006), we looked at two broad sectors for its use, recombinant human collagen and collagen used in sealant, graft, neural and urological applications.
But a review of medical applications of collagen actually should start with dermal fillers because of the long history of use (more than 20 years) in this area, to smooth contour deficiencies.
Dermal fillers
While the demand for facial cosmetic surgery for ameliorating the signs of aging is increasing, many plastic surgeons and dermatologists have become disenchanted with the use of collagen injections because of its bovine origin, need to pretest for an allergic response (by 2% to 3% of the population) and short duration of action (resorbing in 4 to 6 months).
Zyderm and Zyplast are injectable bovine Type I collagen dermal fillers from Inamed (Santa Barbara, California), renamed Allergan Medical after its acquisition this year by Allergan (Irvine, California). These products dominated the dermal fillers market until Inamed introduced CosmoDerm and CosmoPlast injectable grown from human cell cultures using dermal fibroblasts obtained from foreskins.
These fillers do not require pretesting for an allergic reaction. Zyderm and CosmoDerm are non-cross-linked products used to treat superficial wrinkle lines, whereas Zyplast and CosmoPlast are crosslinked and are used to treat more pronounced wrinkles.
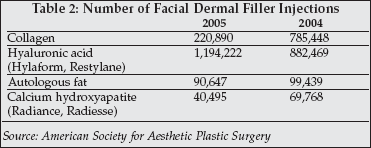
The dermal fillers market is shifting from collagen to hyaluronic-acid based products which have been widely used in Europe for many years but are relatively new to the U.S. market (see Table 2). They include Restylane and Perlane (awaiting FDA market clearance), licensed by Medicis Pharmaceuticals (Scottsdale, Arizona) from Q-Med (Uppsala, Sweden) and Inamed's Hyalform and Captique (resorbs in 3-6 months), licensed from Genzyme (Cambridge, Massachusetts) and Juvéderm (absorbs in 6-12 months), its newly approved hyaluronic acid-based dermal filler licensed from LEA Derm (Paris). The hyaluronic acid-based dermal fillers differ in their method of manufacture (either extracted from rooster combs or produced by a microbial process), in the concentration of hyaluronic acid and their cross linking.
 |
Johnson & Johnson (J&J; New Brunswick, New Jersey) is adding new life to the collagen dermal filler market with Evolence, a collagen-based dermal filler marketed in Canada, Europe, Japan and Israel. It came with the recent acquisition of ColBar Life Science (Herzliya, Israel). Its patented GlyMatrix technology, lasting for 12 months after injection, is based on a biochemical process called glycation that uses sugars to crosslink collagen molecules to produce a matrix that can be tailored to deliver a range of products of varying properties and longevity after injection.
Evolence Breeze, J&J's newest product, is for mid to upper dermis wrinkle correction and is CE marked. Evolence is porcine-derived collagen not requiring sensitivity testing prior to administration.
Artes Medical (San Diego) markets ArteFill injectable dermal filler outside the U.S. It consists of 75% purified bovine collagen and 25% polymethyl methacrylate microspheres suspended within the collagen carrier. Unlike collagen alone, it serves as a permanent dermal filler because the microspheres stimulate the natural development of human collagen. In 2004, the company received an approvable letter from the FDA for its premarket approval application for ArteFill.
Isolagen (Exton, Pennsylvania) is waiting for FDA approval of its protocol to begin a Phase III trial of its dermal filler that is made from a person's own collagen-producing cells (fibroblasts). A specimen is taken from behind the person's ear from which new autologous fibroblast living cells are propagated. Other medical applications under consideration are for treating burns and in dentistry.
Albiorex International (Palm Harbor, Florida) is developing Humallagen, an injectable collagen for use as a dermal filler extracted from human placentas, composed of equal parts Type I and Type III collagen. Type III collagen is naturally cross-linked, is more slowly resorbed and is claimed to persist for a least a year. It is in clinical trials for use in correcting facial wrinkles. The company has patented collagen sealants and biosurgical products that adhere to moist wounds and also prevent adhesion formation.
Surgical Specialties (Reading, Pennsylvania), acquired in March by Angiotech Pharmaceuticals (Vancouver, British Columbia), has an exclusive worldwide distribution agreement from Collagen Matrix Technologies (Boca Raton, Florida) for Dermalogen, an injectable dermal filler that is a human collagen matrix derived from pooled human cadavers from accredited tissue banks. The company is seeking FDA clearance for Dermalogen which was previously being developed by the now-defunct Collagenesis. Surgical Specialties markets absorbable gut sutures, both plain and chromic (treated to reduce absorption rate). These bovine-derived products are made primarily of collagen and used mostly in dental surgery. Gut sutures cannot be sold in Europe because of the fear of mad cow disease.
Other products marketed in the U.S. as injectable dermal fillers are: Cymetra, a micronized form of Alloderm, an acellular freeze-dried dermal graft harvested from human cadavers from accredited tissue banks, from Lifecell (Branchburg, New Jersey); Fascian, preserved and compressed fascia particles prepared from the fascia of cadavers, from Fascia Biosystems (Los Angeles); and Radiesse, calcium hydroxyapatite microspheres in a carboxymethyl cellulose gel from BioForm Medical (San Mateo, California).
Orthopedic and dental applications
DePuy (Warsaw, Indiana), a subsidiary of J&J, markets Healos bone graft replacement, a resorbable hydroxyapatite-coated collagen microfiber with a structure and composition similar to bone. The company is exploring the use of a collagen matrix for delivering bone growth factors. Orthovita (Malvern, Pennsylvania) markets Vitoss, a porous and resorbable bone void filler composed of 80% beta tricalcium phosphate and 20% collagen. Kensey Nash (Exton, Pennsylvania) sells Vitoss scaffold foam, a resorbable beta-tricalcium phosphate manufactured by Orthovita (Malvern, Pennsylvania) that uses Kensey Nash's collagen technology to form a pliant, compression resistant scaffold for use as a bone void filler.
ReGen Biologics (Franklin Lakes, New Jersey/Baar, Switzerland) sells in Europe the CMI collagen scaffold for meniscus repair. It is not approved for sale in the U.S. Implant Innovations (Palm Beach Gardens, Florida), a subsidiary of Biomet (Warsaw, Indiana), sells Ossix collagen membrane for guided tissue regeneration and for restoring bones in the oral cavity. It is licensed from J&J subsidiary ColBar Lifesciences. Osteohealth, a division of Luitpold Pharmaceuticals (Shirley, New York), markets Bio-Gide, a resorbable bilayer membrane for guided tissue regeneration and for filling periodontal defects that is made from purified porcine Types I and II collagen. Bio-Oss, a natural bone grafting material containing 10% purified porcine collagen, is used as a bone void filler in dental surgery. It is licensed by Geistlich & Sons (Wolhusen, Switzerland). Collagen Matrix (Franklin Lakes, New Jersey) sells a resorbable collagen membrane for guided tissue regeneration and, in ridge augmentation procedures, porous collagen used for wound healing after oral surgery, and a fibrillar collagen dental dressing used in areas not easily accessible, such as extraction sites. It presented at the recent annual meeting of the North American Spinal Society (LaGrange, Illinois) a porous and resorbable collagen-mineral composite bone graft available in strip, pad and granular forms, and a resorbable and conformable collagen protective sheet used for bone healing.
Zimmer (Warsaw, Indiana), under license from Integra LifeSciences (Plainsboro, New Jersey), sells BioMend, an absorbable collagen membrane for guided tissue regeneration, and CollaCote, CollaTape and CollaPlug absorbable wound dressings used in dental surgery. Stryker (Kalamazoo, Michigan) sells TissueMend, a remodelable collagen scaffold for surgical repair and reinforcement of soft tissues during rotator cuff surgery, licensed from TEI Biosciences (Boston).
EnColl (Newark, California) markets dental and orthopedic products outside the U.S. made from its ultrapure bovine collagen. They are: Healiguide, a collagen membrane for guided tissue regeneration; and Osseograft, a demineralized bone-derived collagen; and Osseomold, a mixture of calcium sulfate and bioresorbable collagen, bone void fillers, and Periodontal Plus AB, resorbable collagen fibrils containing tetracycline for treating gum disease. The company also sells Enco Skin, a collagen cream for use on wrinkles and photodamaged skin and collagen-coated multiwell plates for use in accelerating cell culture processes.
Angiotech acquired NeuColl (Los Gatos, California), which is developing Collagraft, a synthetic bone substitute comprised of collagen, hydroxyapatite and tricalcium phosphate. It currently is sold in Europe. Zimmer has marketing rights for Collagraft in the U.S. and Japan.
Collagen as a drug delivery vehicle
Innocoll (County Westmeath, Ireland/Ashburn, Virginia) is a pharmaceutical company focused on the local treatment of pain and infection. Its CollaRx collagen sponge and LiquiColl technology platforms are used for targeted drug delivery. Its lead product is Collatamp G which is gentamicin in the CollaRx sponge. It is used as a preventive and treatment of post-surgical infection. The product is approved in 42 countries but not in the U.S. Innocol's pipeline includes a gentamicin-collagen dressing that is in a Phase II trial for the treatment of ulcerated wounds and the CollaRx sponge with a locally-acting anesthetic for the treatment of post-surgical pain.
Oasis (Glendora, California) markets the Soft Shield line of line of collagen corneal shields that are fabricated from porcine scleral tissue and have varying dissolution rates and are used for ocular drug delivery.
Wound dressings and hemostats
The wound care category features the widest range of collagen-containing products. This includes hemostats, artificial skin, composite dressings and sponges.
Integra Lifesciences (Plainsboro, New Jersey) manufactures Dermal Regeneration Template for repair of skin defects. It consists of two layers, a thin collagen-glycosaminoglycan sponge and a silicone membrane. The sponge layer is placed in contact with the wound and serves as a template for the growth of dermal tissue.
Integra Wound Matrix Dressing is a porous matrix of crosslinked bovine tendon collagen and glycosaminoglycan. Collagen Matrix (Franklin Lakes, New Jersey) sells matrix collagen wound dressings in particulate, sponge and film forms. Its leading products are in the neurological and dental specialties. Orthovita (Malvern, Pennsylvania) markets Vitagel, a collagen/thrombin suspension that is used as a surgical hemostat.
Wound Care Innovations (Ft. Lauderdale, Florida) sells CellerateRX activated collagen in powder and gel forms. The product is a Type I hydrolyzed bovine collagen used as a wound filler. It also is sold under the hyCure name for veterinary use.
MPM Medical (Irving, Texas) makes Regenecare wound gel, containing collagen (humectant), aloe (moisturizer), vitamin E (antioxidant) and lidocaine (topical anesthetic).
Davol (Cranston, Rhode Island), a division of C.R. Bard (Murray Hill, New Jersey), markets markets Avitene microfibrillar collagen hemostat, in fibrous and flour forms, and the Ultrafoam collagen sponge.
Xemax Surgical Products (Napa, California) is the maker of Collastat hemostat used in dental surgery and for guided tissue regeneration.
Celgene Cellular Therapeutics (Cedar Knolls, NJ), a division of Celgene (Summit, New Jersey) manufactures Biovance collagen-based decellularized and dehydrated wound covering derived from the human placenta (amniotic membrane) of a full-term pregnancy. It contains natural biologically active substances: Types I, III and IV collagen, fibronectin, glycosaminoglycans, growth factors, hyaluronic acid and laminin.
Organogenesis (Canton, Massachusetts) makes Apligraf skin replacement for burns and wounds care, a bilayered product that consists of a dermal layer composed of human fibroblasts derived from neonatal foreskin in a bovine Type I collagen matrix and an epidermal layer formed by human keratinocytes. VCT-01 is the next generation bioengineered skin that uses a human (rather than bovine) matrix. Revitix is a topical cosmetic product used to reduce the appearance of fine wrinkles and improve skin texture. FortaDerm is a collagen-based antimicrobial wound dressing.
Covalon Technologies (Mississauga, Ontario) markets ColActive, a porcine collagen-based wound dressing and a silver-containing collagen antimicrobial gel sheet made from hydrated denatured porcine collagen.
BioCore Medical Technologies (Elkridge, Maryland) manufactures Medifil particles, pads and gels containing collagen and used on a variety of wound types. Skin Temp is a porous collagen membrane attached to a non-adherent backing for wound care.
Southwest Technologies (North Kansas City, Missouri) sells Stimulen bovine collagen as a powder, lotion, sheet and in an amorphous form for use on various wound types.
TEI Biosciences makes PriMatrix, a acellular dermal tissue matrix comprised of Types I and II collagen and derived from fetal bovine dermis, is used as a wound dressing. It competes against the Oasis acellular wound dressing derived from porcine small intestinal mucosa sold by Healthpoint (Fort Worth, Texas) under license from Cook Surgical (Bloomington, Indiana). TEI also sells SurgiMend, a a remodelable collagen scaffold derived from fetal bovine dermis that is used as a soft tissue patch for the surgical repair of damaged or ruptured soft tissue membranes.
EnColl (Newark, California) sells Helicoll collagen sheets for use on second degree burns and ulcerated wounds, and Healifil, a fibrillar wound filler.
Medical Nutrition USA (Englewood, New Jersey) uses hydrolyzed collagen in Pro-Stat, a liquid protein supplement marketed to dietitians at nursing homes and shown in a clinical trial to be effective in treating malnutrition and healing pressure ulcers.
Potential future applications
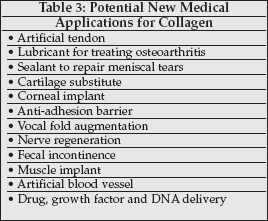
The development of new applications for collagen-based medical products has slowly evolved during the past decades.
There remain many potential new possibilities for collagen in diverse medical specialties as listed in Table 3.
 |
