CDU Contributing Writer
CHICAGO — Cardiovascular disease — the leading cause of death in the U.S. and a major cause of morbidity — also comprises one of the top components of healthcare cost, not only in America but also in most developed countries. As these costs have continued to spiral upward, pressures to limit the cost burden due to cardiovascular disease have increased, driving efforts by physicians and healthcare administrators to develop new approaches to controlling the cost of healthcare.
These conflicting pressures were discussed here by Raymond Gibbons, MD, president of the American Heart Association (AHA; Dallas), at the opening session of the association's Scientific Sessions. Gibbons noted that healthcare spending now accounts for 21% of the federal budget, and healthcare expenses for U.S. corporations are likely to exceed net profits by 2008.
While limiting access to healthcare is one possible solution to address rising costs, there is a general feeling that wholesale rationing of healthcare is not likely to be accepted by most Americans. However, an alternative now beginning to materialize within the healthcare system — including the segment involved with cardiovascular disease — is more selective use of costly diagnostic and therapeutic modalities.
Ultimately, personalized medicine may provide the most effective approach for tailoring the utilization of healthcare technologies to optimize cost as well as outcome. For now, though, there is an increasing emphasis on more selective use of medical technology, particularly for new, high-cost technologies such as drug-eluting stents (DES), implantable cardioverter defibrillators (ICDs) and advanced diagnostic imaging technologies.
DES sees decline
Certainly, other factors have recently played a role in limiting the utilization of DES and ICDs, such as safety and reliability issues that have arisen concerning these technologies. The issue of late stent thrombosis has resulted in a decline in the proportion of patients receiving DES devices in the U.S., from a level of more than 90% of percutaneous coronary intervention (PCI) procedures in many centers to levels of 80%-85%. Even more selective use of DES has been advocated by some cardiologists, with a target of 50%-55% proposed by some and currently the prevailing rate in Europe.
Not unexpectedly, the lower utilization level in Europe has been driven less by concerns about device safety and more by issues of costs — both the costs of the devices themselves and also the associated dual anti-platelet therapy required that typically runs about $4 per day and must be continued for three to six months.
In addition, the true extent of the added risk of adverse events for DES compared to bare-metal stents (BMS) has not been definitively quantified, and it ultimately may not prove to justify any reduction in use, particularly as second-generation DES devices become available. But cost issues will remain, and will continue to be a driver for more selective DES use.
Among the approaches being developed to enable better selection of patients for interventional therapy are advanced technologies for plaque characterization, which promise not only to allow matching of device characteristics to lesion type, but also to dictate use of alternative, less costly disease management approaches in some patients.
A focus on interventional
Other advances in interventional therapy highlighted at the AHA sessions included carotid stents, devices for closure of heart defects such as patent foramen ovale (PFO), percutaneous valve implantation and catheter-based treatments for arrhythmia.
A new study presented at the conference also revealed issues with the timely delivery of interventional therapy, finding that 80% of treatment centers in the U.S. fail to initiate therapy within the 90-minute time window specified by the latest guidelines from the AHA and the American College of Cardiology (Washington). Resolving those deficiencies could drive growth in demand for rapid diagnostic technologies to speed patient triage in the emergency room, as well as for improvements in hospital infrastructure to optimize efficiency. A number of simple strategies were identified in the study that can also help reduce treatment delays, such as early activation of the cath lab and more rapid response by cath lab staff when paged.
The type of therapy that is most appropriate for patients with coronary disease continues to be a topic of continuing and significant debate. For coronary artery occlusions or stenoses — the cause of myocardial infarction and angina — interventional treatment using angioplasty and stenting is increasingly the preferred approach. As discussed by Cindy Grines, MD, of William Beaumont Hospital (Royal Oak, Michigan) at a symposium held in advance of the AHA sessions, about two times as many patients who present with ST-segment elevation myocardial infarction (STEMI) are now treated with primary angioplas-ty/stenting as are treated with thrombolysis, which was at one time the preferred modality.
Recent studies have demonstrated improved outcomes for primary PCI, regardless of the delay in starting therapy. In fact, studies have shown that there is no advantage to giving thrombolytic therapy before the patient arrives at the hospital if primary PCI is performed, and also no advantage in adding thrombolysis to PCI (so-called facilitated PCI). Rather, mortality rates after treatment are somewhat higher for patients treated with facilitated PCI.
Instead, Grines recommended giving anti-coagulants such as clopidogrel when a STEMI patient arrives at the emergency department, and then proceeding to PCI as quickly as possible. Other approaches to improving outcome for myocardial infarction patients, such as removal of thrombus via clot maceration and aspiration, or the use of filters or occlusion balloons during PCI to avoid embolization due to debris created during the procedure, have produced disappointing results.
In the case of DES vs. BMS
The use of DES versus bare-metal stents (BMS) is another area of controversy that was discussed in numerous sessions at the AHA conference. The issue of late-stent thrombosis with DES has become one of the most widely debated topics in interventional cardiology in 2006, and it was pushed to the forefront with the presentation of a metanalysis on the subject earlier this year at the World Congress of Cardiology Conference (WCC) in Barcelona, which was highly critical of the early claims for DES.
Recent downturns in DES use already indicate that cardiologists in the U.S. are re-evaluating the broad adoption of DES. As shown in Table 1, DES utilization in the U.S. has dropped from almost 90% of PCI procedures in the first half of 2006 to an estimated 80% level now. Utilization in regions outside the U.S. has not declined, although the rate of increase in utilization has slowed.
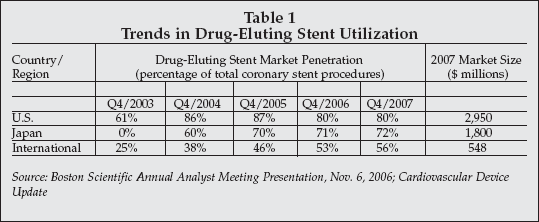 |
According to some suppliers of DES, such as Boston Scientific (Natick, Massachusetts), the No. 2 player worldwide, utilization is likely to rebound in 2007, finishing the year at 85% in the U.S. and continuing to increase slowly elsewhere.
However, that prediction is not shared by all cardiologists. For instance Martin Leon, MD, of Columbia University Medical Center (New York), one of the earliest proponents of DES use, has projected that the DES utilization rate in the U.S. could drop to 80% by 2008.
Cordis (Miami Lakes, Florida), a unit of Johnson & Johnson (New Brunswick, New Jersey), the global DES market leader, released the results of its analysis of late stent thrombosis with the Cypher stent in a Nov. 8 webcast, just prior to the start of the AHA conference, showing no excess in the rate of thrombosis for the Cypher compared to BMS when only definite or probable thrombosis incidents are included.
Other analyses included adverse events not definitively shown to be due to a cause other than stent thrombosis within the definition of late stent thrombosis. Nevertheless, it is clear that many cardiologists are re-evaluating their criteria for deciding when to use a drug-eluting stent vs. a bare metal stent.
In STEMI patients, for example, the use of DES provides little added benefit compared to BMS because rates of restenosis in those patients are already quite low (5%-7%) when BMS are used.
DES use is not only more costly but indications are that DES devices also may expose the patient to a long-term risk of thrombosis.
In elective PCI procedures in non-MI patients, there are definite benefits in reduced restenosis rates with DES, but this comes with the added risk of late thrombosis as well as the cost of extended dual anti-platelet therapy, estimated at $1 billion annually at current levels of DES utilization.
Second-generation DES in position
The situation could change in the future as second-generation devices enter the market designed to reduce or eliminate the drawbacks of existing DES.
As discussed by Patrick Serruys, MD, PhD, of the Thoraxcenter (Rotterdam, the Netherlands), a number of devices are showing promise in clinical trials, including the CoStar from Conor Medsystems (Menlo Park, California), a company that is slated to be acquired by J&J (see story p. 8); a new version of the absorbable metal stent (AMS) from Biotronik (Berlin), designed with drug-eluting reservoirs; the Axxion drug-eluting stent from Biosensors (Singapore), employing a bioabsorbable drug-eluting coating and already in use clinically outside the U.S.; a DES that is dual drug-loaded with he-parin and siroli-mus from Sahajanand Medical Technologies (Gujarat, India); and additional devices from Sorin Biomedica (Saluggia, Italy), Xtent (Menlo Park, California) and Terumo Medical (Tokyo).
A trend seen in a number of the second-generation devices is the use of bioabsorbable polymers to avoid the adverse inflammatory effects of existing durable polymer coatings. Another approach, exemplified by the Genous R-Stent from Orbus Neich (Hong Kong/Hoevelaken, the Netherlands), uses a bioactive coating to attract endothelial cells to the stent surface, in principle producing a non-thrombogenic, biocompatible surface on the stent within 48 hours of implant. That strategy, however, has limitations related to the level of circulating endothelial progenitor cells in the patient's bloodstream.
Devices such as the Conor stent are attractive because of the ability to employ multiple approaches to overcome the problems with first-generation devices, such as using bioabsorbable polymers as well as multiple drugs. An important change in development strategy for next-generation DES is the focus on improving safety and biocompatibility, rather than on further reduction in the rates of restenosis.
New strategies vs. thrombosis
One new tactic for reducing late stent thrombosis events in patients receiving DES implants was described by Matthew Price, MD, of Scripps Clinic (La Jolla, California), discussing a study that used the VerifyNow point-of-care assay system from Accumetrics (San Diego, California) to determine the responsiveness of stent patients to clopidogrel, the primary anti-platelet drug used to prevent thrombosis.
The standard of care for DES patients now includes three to six months of therapy with clopidogrel and aspirin after the implant procedure. However, a significant percentage of patients, ranging from 5% to 40%, do not respond to clopidogrel, and a similar percentage does not respond to aspirin. Price found that three of the four patients who had a stent thrombosis event were non-responsive to clopidogrel. All four patients were taking clopidogrel at the prescribed dose at the time of thrombosis.
Price said the study shows that non-responsiveness to clopidogrel is a cause of stent thrombosis, and he said he believes that a large-scale randomized study should be performed to assess the use of the VerifyNow P2Y12 assay in titrating clopidogrel treatment.
He is also conducting a follow-up study to determine if increasing the dose of clopidogrel in non-responsive patients will reduce the rate of stent thrombosis. Information from anti-platelet drug response testing could also be used along with other patient characteristics to decide whether to use BMS or DES.
Valve repair and reconstruction
A number of presentations at the AHA sessions also covered the latest developments in minimally invasive (MIS) heart valve repair, as well as interventional treatment of heart defects such as PFO, atrial septal defects, and ventricular septal defects.
Thomas Walther, MD, of the Heart Center (Leipzig, Germany), described initial experience with the Cribier-Edwards Ascendra prosthetic aortic valve implanted via a minimally invasive transapical approach (TAP-AVI). The device is one of a number of new valves under development by Edwards Lifesciences (Irvine, California) that can be implanted using MIS techniques. Walther presented the results of a feasibility study using the device in 34 high-risk patients.
The implant procedures were performed in a hybrid operating room by cardiac surgeons, using a mini-thoracotomy to provide access for the 40 Fr sheath required by the Ascendra device, which has a diameter of 25 mm. The procedure was performed on the beating heart, using extracorporeal circulation in 16 patients.
The pericardial xenograft valve is fixed within a 16 mm-long stainless steel balloon expandable stent. Good valve positioning was achieved in 93% of the patients, with the remainder requiring conversion to open surgery. Total procedure time was six hours.
Twelve patients had mild perivalvular leaks, two had moderate leaks, and one had a severe leak. Although mortality at 30 days was 13.6%, that rate was considered normal, based on the risk profile of the patients treated.
The main contraindication for use of the Ascendra valve is calcification of the native annulus. The surgeons are now advocating that Edwards initiate a randomized controlled trial of the device, and believe that the potential target patient population could be very large.
The existing market for prosthetic heart valves used in treatment of all types of heart valve disorders will exceed $1 billion worldwide in 2006, and Edwards estimates that up to 50% of individuals with aortic valve disorders are currently not treated.
John Carroll, MD, University of Colorado Health Science Center (Denver), discussed the current status of development of devices for interventional treatment of heart defects such as PFOs, atrial septal defects, and ventricular septal defects. Five companies now market devices for interventional repair of heart defects including NMT Medical (Boston), AGA Medical (Golden Valley, Minnesota), W.L. Gore (Flagstaff, Arizona), Cardia (Eagan, Minnesaota), and St. Jude Medical (St. Paul, Minnesota). In addition, Cierra (Redwood City, California) is developing the PFX Closure System, which employs transcatheter delivery of radiofrequency energy to weld heart tissue at the site of a PFO and effect closure.
The potential worldwide patient population for devices used to treat heart defects numbers in the millions, as shown in Table 2. More than 20,000 PFO closures have been performed using NMT Medical's CardioSEAL and STARFlex devices. The CardioSEAL price was set at $5,500 in the U.S. under a Humanitarian Device Exemption (HDE), but the HDE was voluntarily withdrawn recently because actual utilization in the U.S. has surpassed the level of 4,000 patients per year, the maximum allowed for an HDE device.
 |
NMT is developing the BioSTAR, a bioabsorbable porcine collagen PFO closure device comprised of extracellular biomaterial that promotes cell growth and a cobalt metal framework. The biomaterial is replaced by native tissue over a period of about two years after implant, leaving only the metal framework as a permanent implant.
That not only minimizes the potential risk associated with the presence of any life-long implant, but also leaves open the possibility to perform a percutaneous valve implant procedure in the same patient if that should become needed.
The FDA has recently allowed a change in protocol for the on-going MIST II trial for use of the BioSTAR. The endpoint of the trial was also altered to reduction, rather than complete elimination, of migraine headache. Worldwide sales of the CardioSEAL and STARFlex devices increased 12.8% in 2005 to $19.3 million.
Target: vulnerable plaque
The detection of vulnerable plaque — or intra-arterial plaque that is prone to rupture and cause a myocardial infarct — continues to be a topic of research focus in cardiology and it has attracted significant investment within the medical device industry. The goal is to develop techniques, ideally non-invasive ones, that can not only detect the presence of vulnerable plaques but also can characterize the plaque to allow physicians to decide upon the most appropriate therapy — ranging from PCI with DES implantation to medical and diet/exercise therapy aimed at prevention of rupture at a specific high-risk site, or modifying the lipid content of plaque as a generalized approach to risk reduction.
Targeted molecular imaging is one increasingly promising approach for characterization of vulnerable plaque, as described by Gang Bao, of Emory University (Atlanta). Bao described a technique using DNA-targeted nanoparticles for in vivo plaque characterization. The particles also carry a toxin on their surface that create micropores in cells, allowing the DNA to penetrate to the cell nucleus and bind to specific molecular targets characteristic of macrophages, cell adhesion, endothelial activation and shear stress, all of which are potential indicators of vulnerable plaque.
Feasibility studies in animals have shown a correlation between expression of cell adhesion molecules by cells in the inner vessel wall and shear stress, which is believed to be a precursor to plaque development. At present, Bao is using a fluorescence quenching method to detect selective binding of the particles. However, magnetic nanoparticles have also been used that could potentially be used for MR imaging of plaque.
Mark Whooley, MD, of Stanford University (Stanford, California), also is developing noninvasive MRI techniques for vulnerable plaque detection. Whooley is using multi-functional nanoparticles formed from synthetic polymers and labeled with gadolinium for MR detection. By varying the polymer composition, the particles can be designed to distribute selectively to blood or various tissues such as the liver. Whooley has selectively imaged injured segments of carotid arteries using MRI.
The sensitivity of the technique is heightened by the enhancement of the gadolinium signal when it is complexed to the polymer. However, refinement of the technique is needed before reliable selective imaging in humans can be achieved.
Nanoparticles get magnetic attraction
Farouc Jaffer, of Massachusetts General Hospital (Boston), described the use of dextran-coated magnetic nanoparticles coated with a molecule that specifically binds to activated macrophages, such as are found in vulnerable plaque. The Activated Macrophage Targeted Agent (AMTA) has been used in a clinical study and shown to selectively localize in regions where activated macrophages are present. A clinical trial has been launched to evaluate changes in the distribution and number of activated mac-rophages in patients treated with statins to reduce vascular inflammation.
Another version of the agent has been labeled with a cell adhesion molecule (VCAM-1) to preferentially image endothelial cells. And still another agent has been developed that targets proteases that are known to be involved in atherogenesis. Reductions in protease activity in vascular tissue have been demonstrated in studies of animals treated with statins.
Jaffer also has developed a new catheter that can be used to perform near-infrared fluorescence imaging that has been used in animal studies to detect regions of protease activity associated with atherosclerotic injury. The goal is to use targeted fluorescence imaging to identify plaques that are likely to cause atherothrombotic complications such as myocardial infarction.
Molecular imaging agents targeting macrophages for vulnerable plaque imaging are under development by early-stage company NanoScan Imaging (Landsdale, Pennsylvania). As described by Fabien Hyafil, of Mt. Sinai School of Medicine (New York), a targeted CT contrast agent, N1177-iv, is under development by NanoScan that provides image enhancement of atherosclerotic plaques. The iodine-containing agent is given intravenously, and has been shown to specifically target lipid-rich and macrophage-rich plaques, although so far the nature of the macrophage-specific marker has not been determined. The agent is injected intravenously, and after a two-hour waiting period imaging is performed.
According to Hyafil, the ability of N1177-iv to selectively enhance local concentrations of macrophages in CT images of the vessel wall may be due to the effects of macrophage enzymes on the solubility of the agent. N1177 is also being developed for applications in cancer imaging, and has the obvious advantage of not requiring catheterization while still providing a high-resolution image of the arteries.
As shown in Table 3, the number of individuals in the U.S. with coronary heart disease who would be candidates for imaging with an agent such as N1177-iv will total more than 14.5 million by 2010, the earliest date targeted by the company for market introduction. The target patient pool is projected to increase to 15.4 million by 2015.
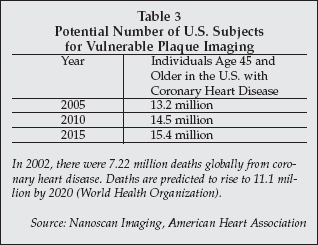 |
Evaluating plaque density
Shigeki Kimura, MD, of Tsuchiura, Japan, reported results of a study that tracked the correlation of density of plaque imaged via CT with histological analysis of the plaque tissue after it was removed by directional coronary atherectomy. The study evaluated plaques in acute coronary syndrome (ACS) patients as well as in patients without acute coronary syndromes.
As determined by the CT imaging, plaque density was significantly lower in lipid-rich plaques, the plaques believed to be most prone to rupture, compared to fibrous or mildly calcified plaque. More low-density plaque was also found in ACS patients, consistent with the concept that lipid-rich plaques are the ones most likely to rupture and cause a myocardial infarction.
Another approach in the use of CT to non-invasively detect vulnerable plaque was described by Cynthia McCollough of the Mayo Clinic (Rochester, Minnesota). In collaboration with Siemens Medical Solutions (Malvern, Pennsylvania), McCollough is assessing a new dual-energy scanner that allows discrimination of different metals, particularly iron and calcium, in CT images. Because iron is believed to be present in higher concentrations in microhemorrhagic plaque containing excess blood, identification of regions of high iron content in plaque could allow vulnerable plaques to be detected prior to rupture, but when the risk of rupture is high.
The Siemens scanner used in the study is FDA-cleared for dual-energy imaging, but the software used for iron and calcium discrimination is still a work in progress. So far, McCollough said she has demonstrated the ability to discriminate iron and calcium in model mixtures and is now proceeding with animal studies.
CT is uniquely suited for making some measurements, since it provides the spatial resolution needed to separate the iron signal produced from blood flowing in the vessel versus iron from blood in microhemorrhages in the vessel wall.
More plaque imaging strategies
A number of other technologies were also described at the AHA sessions that are showing promise for detection and analysis of vulnerable plaque, including intravascular ultrasound, intracoronary thermography, and intravascular optical coherence tomography. These technologies, however, all require an invasive catheterization procedure, making them unsuitable for use as a general screening technique. Instead, such techniques would probably be used after a suspect region of plaque had been detected using a non-invasive molecular imaging modality.
Ultimately, a three-stage triage process is likely to emerge that will consist of initial screening using a panel of risk markers analyzed via in vitro diagnostic tests, such as a lipid profile, markers of vascular inflammation, and perhaps genetic tests for specific disorders predictive for coronary artery disease, coupled with physiological tests such as blood pressure measurement. Individuals identified at elevated risk in the initial screen would then be evaluated using noninvasive molecular imaging to determine if suspect plaques are present, and their location in the coronary arteries.
If a positive result is obtained via imaging, the next step would be catheterization and inspection of the suspect plaques with a transcatheter modality to confirm the nature of the plaque and decide upon the most appropriate therapy.
