BBI Contributing Editor
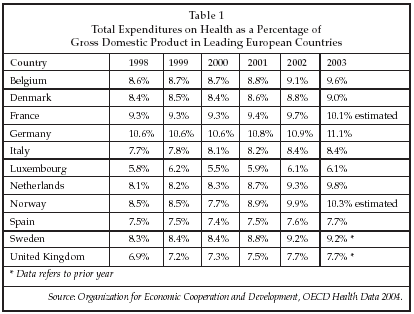
STOCKHOLM, Sweden – The European market for cardiovascular devices has historically grown at a somewhat slower rate than the market in the U.S., due in large part to the more stringent controls on healthcare expenditure in Europe. Those more restrictive controls have in turn resulted from slower economic growth in most of the major Western European countries. Lately, however, growth has begun to accelerate as the percentage of GDP devoted to healthcare has begun to increase in most of the leading countries, as shown in Table 1 below. While rates of healthcare spending in Europe remain well below those in the U.S., there nevertheless is a trend toward increased spending by governments as well as private individuals and insurers.
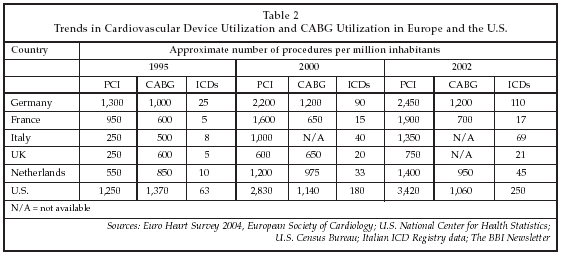
In the arena of cardiovascular disease treatment, as shown in Table 2 on below, procedure volumes in Europe have begun to expand rapidly within the past one to two years in certain segments, such as ICD implants and percutaneous intervention. Correspondingly, the markets for those products are also exhibiting strong growth, although high prices for products such as drug-eluting stents, at least as perceived by hospital users in Europe, have continued to inhibit more widespread adoption. The latest trends in device utilization in Europe, as well as new devices and technologies under development, were key topics here at the early September annual meeting of the European Society of Cardiology (ESC, Sophia Antipolis, France).
In addition to drug-eluting stents, other technologies highlighted at the ESC congress included non-invasive cardiovascular imaging, telemedicine and remote diagnosis in cardiovascular disease, ablation of atrial fibrillation, percutaneous repair and replacement of heart valves, device-based closure of congenital heart defects, and the use of cellular therapy to treat heart disease and stroke. Cost-effectiveness of new medical technologies also is an important topic throughout Europe, particularly in countries such as Germany, where pressures to control costs have been among the most intense of any country worldwide. As an indication of the growing interest in cost containment, reprocessing of single-use medical devices attracted considerable interest at the ESC conference as an attractive option for hospitals to reduce the cost of medical supplies.
Device-based treatment expanding
The expanding use of interventional therapy within the cardiology community in Europe is demonstrated by the most recent data from the Euro Heart Survey, a survey of hospitals in Europe that has been performed at varying intervals since 1990. This year, the survey has been re-structured as a continuously updated assessment tool covering 32 European countries that is available to sponsors and ESC members on-line. As shown in Table 2, while the utilization of percutaneous coronary intervention has increased substantially in Europe, doubling or tripling in many cases between 1999 and 2002, coronary artery bypass graft surgery has stabilized, and in some cases declined. Total PCI procedures in Europe are estimated at approximately 600,000 per year, according to data from stent suppliers. Compared with the U.S., utilization of PCI and ICDs remains low in Europe, with the rate per million inhabitants at less than half the U.S. figures. Based on existing trends, it is unlikely that the rates in Europe will reach those in the U.S. within the foreseeable future.
Drug-eluting coronary stents are playing a growing role in the cardiovascular device market in Europe. While utilization is lower than in the U.S., some centers are using drug-eluting stents for every PCI patient, whereas others use the devices for less than 20% of patients. Utilization varies from country to country, with Germany having one of the lowest rates, quoted at 14% as of 4Q04 by Boston Scientific (Natick, Massachusetts), while rates in Denmark, Switzerland and Portugal are 50% to 70%. The overall rate in Europe as of 4Q04 is estimated at 35%. The availability of reimbursement is a key factor determining the level of utilization in a country. A decline in drug-eluting stent prices is another factor responsible for increased use, which, however, results in a less-than-proportionate increase in the dollar volume market. The average cost of a Cypher stent from the Cordis (Miami Lakes, Florida) unit of Johnson & Johnson (J&J; New Brunswick, New Jersey) or a Taxus stent from Boston Scientific is now about EUR 1,400, although it varies by country. Two years ago, the devices cost about EUR 1,800 on average. Cost of the new Endeavor drug-eluting stent from Medtronic (Minneapolis) is only EUR 1,100, demonstrating the long-anticipated decline in prices as a result of increased competition in the market.
Based on the results of the Basel Stent Kosten-Effektivit ts Trial (BASKET), as presented at the ESC conference by Dr. Matthias Pfisterer of University Hospital (Basel, Switzerland), drug-eluting stents already are cost-effective for between two-thirds and three-fourths of patients. That study assumed that the initial cost for drug-eluting stent treatment was EUR 1700 higher than for treatment with bare-metal stents, based on the use of two drug-eluting stents per patient.
Over time, much of that extra cost is recovered due to the reduced need for re-intervention with drug-eluting stents, particularly for stenting of small (2.5 mm or less) vessels and other types of high-risk lesions. Based on existing prices for bare-metal stents of about EUR 400, the cost differential for drug-eluting stents already is at a point that makes their use cost-effective for most patients. Furthermore, competition will undoubtedly increase in the European market, resulting in continued price declines.
At least three new drug-eluting stents – the Coroflex Please from B. Braun Melsungen (Melsungen, Germany), the Pico Elite from AMG Internati-onal (Raesfeld-Erle, Germany) and the Janus stent from Sorin Biomedica Cardio (Saluggia, Italy) – are poised to receive regulatory clearance to enter the market in Europe soon, and a total of 16 new devices are pending receipt of the CE mark. The newest device to receive a CE mark is the Liberte drug-eluting coronary stent from Boston Scientific.
In addition, the Genous stent, a new device from OrbusNeich (Hoevelaken, the Netherlands) that received a CE mark in late August, while technically not a drug-eluting stent, will compete in the market in Europe. Its endothelial progenitor cell coverage technology, which results in complete coverage of the stent with endothelial cells 48 hours post-implant, is designed to minimize restenosis, as are drug-eluting stents, while avoiding certain problems of currently marketed devices related to inflammation and stent thrombosis.
The company recently discovered in the HEALING 2 trial that a high degree of patient-to-patient variability in outcome was attributable to differences in the level of endothelial progenitor cells in blood. The EPC level was in turn found to be dependent on statin treatment. OrbusNeich will as a result monitor statin levels in the upcoming HEALING 3 trial to determine if maintaining statins at an optimal level will eliminate between-patient differences in endothelial cell coverage of the stent.
Another device employing a similar design concept is under development by Guidant (Indianapolis), and relies upon an RGD peptide coating on the surface of a polymer-covered metal stent to recruit endothelial progenitor cells to rapidly cover the stent after implantation, enhancing biocompatibility and also in principle inhibiting stent-related thrombosis.
Thrombosis of drug-eluting stents was a topic highlighted at a number of sessions at the ESC congress. While late thrombosis in drug-eluting stents occurs at a very low rate of between 0.3% and 1%, the number of affected patients is still significant since millions of the devices have now been implanted worldwide. Although that rate appears to be similar to the thrombosis rate for bare-metal stents, no studies have directly compared the two types of devices. Late thrombosis rates determined in various clinical studies have generally been lower for the Cypher than for the Taxus stent (0.5% vs. 0.8% in one large study conducted by Dr. Antonio Colombo in Italy), although the range was quoted at from 0.2% to 2.8% for Cypher stents vs. 0.2% to1.8% for Taxus by Dr. A. Abizaid of Institute Dante Pazzanese of Cardiology (Sao Paulo, Brazil) at a symposium at the ESC congress. The rate for the new Medtronic Endeavor stent is 0.5%.
Late thrombosis is a particular problem for patients who must stop taking dual antiplatelet drug therapy, a treatment that has been shown effective in preventing thrombosis. A significant percentage of patients who stop their antiplatelet therapy experience stent thrombosis, and when it occurs, late stent thrombosis has a high mortality rate.
In order to avoid late thrombosis, developers of second-generation devices are trying a number of new strategies, such as elimination of polymer coatings to carry the drug; the use of dual drugs, one to prevent tissue ingrowth and a second to suppress inflammation; and bioabsorbable stents. Boston Scientific, Guidant and Biotronik (Berlin) all are developing bioabsorbable stents. The Biotronik device is made of magnesium, and is completely absorbed over a period of a few weeks. In addition to avoiding the use of a polymer, there is no interference with non-invasive imaging once the stent has been absorbed. Another possible benefit is that the elimination of a permanent metal scaffold will allow the artery to remodel naturally and regain the compliance characteristic of a normal vessel, a process that is inhibited when a metal stent remains in place. Animal experiments with the Biotronik absorbable stent have demonstrated positive remodeling of arteries (i.e., additional enlargement of vessel diameter) after the device is absorbed.
Another device, under development by Reva Medical (San Diego) in partnership with Boston Scientific, uses a tyrosine-derived polycarbonate copolymer that can be loaded with a drug such as paclitaxel to prevent restenosis and then bioresorb once the artery has healed. Guidant’s device will combine a bioabsorbable scaffold with the anti-restenosis drug everolimus.
Another next-generation stent, the CoStar from Conor Medsystems (Menlo Park, California), also employs a bioabsorbable polymer, but combines it with a metal stent that incorporates hundreds of small laser-cut holes that can be loaded with drugs. The holes are then covered with a bioabsorbable polymer that dissolves once the stent is implanted, allowing the drugs to be delivered to the vessel wall. A number of different drugs can be loaded into the device, such as an anti-restenosis agent plus an anti-inflammatory agent such as pimecrolimus or melatonin. Data from the EuroSTAR study demonstrated a 2.9% target vessel revascularization rate and no reported cases of stent thrombosis between the cessation of anti-platelet therapy at six-month and 12-month follow-up.
The Janus Carbostent from Sorin Biomedica Cardio embodies yet another approach that avoids the use of a polymer by the use of a biocompatible carbon coating on the surface of a metal stent. The anti-restenosis drug Tacrolimus can be loaded into the coating, and subsequently elutes from the stent into the vessel wall. Results from initial clinical studies show a 6.4% target vessel revascularization rate at six months follow-up.
Occam International/Biosensors International (Eindhoven, the Netherlands) also is also developing a new drug-eluting stent designed for improved biocompatibility. The Occam device employs a glycocalyx coating derived from materials developed for use in hemodialysis. The coating has been shown to remain unaltered over a period of 90 days when exposed to blood, and can be loaded with paclitaxel to provide drug elution capability.
Rapid growth for ICDs
ICDs represent another important and rapidly growing segment of the cardiovascular device market in Europe. As discussed by P. Fiorelli of S. Maria della Misericordia Hospital (Udine, Italy) at the ESC congress, an increasing number of ICDs are being implanted in Europe for prophylactic use. The number of ICD implants in Italy increased 30% per year from 2001 to 2004, and the percentage implanted for prophylactic use increased from 6.5% to 24%.
Nevertheless, utilization of ICDs in Europe lags well behind that in the U.S., indicating considerable potential for growth in the market in Europe. At present, according to data presented by Fiorelli, only 23% of patients who are eligible for an ICD based on a low left ventricular ejection fraction (<30%) receive an implant. The most often quoted reason for not receiving an implant, based on interviews, is that patients are undecided about whether or not to have the procedure. Other reasons include physician denial, poor physical condition, and failure to have an implant even though the patient was referred for the procedure.
A new type of ICD was exhibited at the ESC congress by Cameron Health (San Clemente, California) that could drive expanded adoption because of its ease of use and reduced risk of complications. The new device, the Axiom ICD, uses subcutaneous leads, so that only a small cutdown is required for implantation. That reduces infection risk and simplifies the implantation procedure and in particular eliminates the need to use fluoroscopy, reducing radiation exposure for physicians. In addition, programming of the device is considerably simplified compared to the process required for existing ICDs. The Axiom is under development, with the first controlled clinical study planned to start in January 2006. When launched, the company expects that the device will be priced in the middle of the range existing in the market.
Other developments in the European market for electrophysiology products described at the ESC conference included initial results with a new device for ablation of atrial fibrillation, the Arctic Front catheter from CryoCath (Montreal, Quebec). As discussed by Dr. H.F. Pitschner of the Kerckhoff Klinik (Bad Nauheim, Germany), the Arctic Front uses a new technology, cryoablation, to ablate cardiac tissue for eradication of atrial fibrillation. In an initial study involving 20 patients, 16 of 19, or 84%, were free of atrial fibrillation at 12-month follow-up. An additional patient who experienced recurrent atrial fibrillation was re-treated and was symptom-free at six months after the procedure.
The device features a catheter with a 20 mm balloon at the tip. A console is used to deliver a refrigerant to the tip that vaporizes, creating cryogenic temperatures. The cooled balloon adheres to the cardiac tissue, stabilizing its position during the ablation procedure. The use of a large ablation area and the high degree of positional control of the device allows ablation procedures to be completed rapidly, in less than 2.5 hours. Complications so far have been minimal, and mainly include nerve palsy, which resolves.
Other new technologies discussed at the ESC congress include a minimally invasive device for closure of patent foramen ovale (PFO) under development by Cierra (Redwood City, California) and percutaneous heart valves being developed by Edwards Lifesciences (Irvine, California). The Cierra PFX Closure System employs radiofrequency energy delivered through a catheter to weld the heart tissue at the site of a PFO, providing permanent closure without the need for implantation of a device. The goal of the procedure is to prevent strokes, since PFOs are a leading cause of stroke in young patients. PFOs also have been linked to migraine headaches. The first successful procedure using the PFX system was performed by Dr. Horst Sievert of CardioVascular Center Frankfurt (Frankfurt, Germany) in April. As discussed by Sievert at the ESC congress, the system has so far proven safe and effective, with no deaths or strokes related to the procedure, four cases of tamponade, five small pericardial effusions and three groin complications noted.
Other devices for PFO closure, such as the CardioSEAL and STARFlex from NMT Medical (Boston); the Amplatzer from AGA Medical (Golden Valley, Minnesota); the Intrasept from Cardia (Burnsville, Minnesota); the Premere from Velocimed (Maple Grove, Minnesota), now a unit of St. Jude Medical (St. Paul, Minnesota); and the Excluder from W.L. Gore (Flagstaff, Arizona), all result in placement of a permanent implant in the heart. While radio frequency closure using the PFX system now is applicable to about 70% of patients with PFO, further improvements to the system are expected to make it applicable to a higher percentage of patients. The system can be used regardless of the morphology of the PFO, but is not applicable to small atrial-septal defects. Sievert said he believes the PFX system could replace other types of closure technologies in about 50% of all procedures.
The current status of percutaneous heart valve repair and replacement was discussed by Dr. Alain Cribier of Rouen University Hospital (Rouen, France). Cribier is performing clinical studies with the Cribier-Edwards Percutaneous Valve, a bioprosthesis consisting of three leaflets of equine pericardium sutured to a balloon expandable stainless steel stent. Expansion is performed with a 22 mm balloon supplied by NuMED (Hopkinton, New York), introduced through a 22 Fr sheath. The procedure is performed to treat aortic stenosis, which has a mortality of about 40% at one year following diagnosis if not treated. More than 150,000 open-heart surgeries are now performed each year worldwide to treat the condition. However, abo-ut one-third of patients with aortic stenosis are not candidates for surgery due to their age, the presence of depressed left ventricular function, cancer or renal failure. Percutaneous valve implantation offers an alternative for such patients, who otherwise face a very bleak prognosis.
So far, Cribier has performed the procedure on 40 patients, with successful implantation in 33. Two patients have experienced no valve dysfunction at two years post-implant. However, at least one procedure-related death has occurred, prompting a re-evaluation of the implant technique and leading the in-vestigators to switch to a retrograde, vs. an antegrade, approach. In addition, a recent modification has resulted in an increase in the valve diameter, a change that is expected to ensure against migration of the valve after implant. A new trial involving 100 patients and using the new valve design along with a retrograde approach is about to begin.
Another topic highlighted at the ESC congress of importance to suppliers of medical devices in the European market is reprocessing of single-use devices. Reprocessing has become established in the U.S., where revenues for providers of reprocessing services are now in excess of $100 million per year. In Europe, the market for third-party reprocessing services is about EUR 30 million, and is served primarily by Vanguard AG (Berlin, Germany). Vanguard AG is not a part of Vanguard Medical Concepts (Lakeland, Florida), a company that recently merged with Alliance Medical (Phoenix) to form the largest reprocessor of single-use medical devices in the U.S. Vanguard AG provides reprocessing services for devices such as PTCA catheters and electrophysiology catheters. The company provides services to more than 1,000 customers in Europe, and 90% of the catheterization labs in Germany now are using its services.
The regulations pertaining to reprocessing of single-use devices are similar, but not identical to, those in the U.S. In the U.S., hospitals can save up to 50% of the cost of disposable supplies by switching to reprocessing. Overall, the potential savings are less, since not all devices can be reprocessed. In addition to cost savings, reprocessing is attractive because it minimizes medical waste generation. Along with device reprocessing, Vanguard AG provides other services including logistics, auditing, financing, operating room management and instrument management. Medical device reprocessing is expanding in Europe, as indicated by Vanguard’s expansion into Denmark, Switzerland and Poland.
Advances in diagnosis and monitoring
Other new developments were highlighted at the ESC congress in the areas of patient monitoring outside of the hospital setting as well as non-invasive imaging. Advances in non-invasive imaging are enabling cardiologists to improve detection of cardiovascular disease at an early stage, and to rapidly differentiate cardiovascular disease from other conditions that can produce similar symptoms. For example, the introduction of 64-slice computed tomography (CT) systems from suppliers including GE Healthcare (Chalfont St. Giles, UK), Philips Medical Systems (Best, the Netherlands), Siemens Medical Solutions (Erlangen, Germany) and Toshiba Medical Systems (Tokyo), is enabling non-invasive CT angiography to be used as a rapid method to diagnose patients with chest pain. Previously, using 16-slice systems, capture of a CT angiogram image at sufficiently high spatial resolution of under 0.5mm to identify stenoses that can be detected via conventional angiography required an impractical breath-hold time of 35 to 40 seconds.
With newer 64-slice systems, the breath-hold time is reduced to eight to 10 seconds, making it practical to perform imaging on patients who present with chest pain and other symptoms of myocardial infarction, and to do so within a timeframe that can fit within the requirements for maximum door-to-needle time in the management of acute coronary syndrome pati-ents. For patients who present with ambiguous symptoms, and non-definitive ECG and blood test results, it now is possible to reflex to a non-invasive CT scan rather than proceeding directly to invasive angiography. In addition, a CT scan can allow a wider range of pathologies to be detected as compared to angiography, so that, for example, a patient with a gastrointestinal disorder with clinical symptoms mimicking heart attack can not only be spared invasive angiography, but also can receive a rapid diagnosis of the true cause of symptoms.
The ability of high-resolution CT to analyze the entire coronary vasculature in one exam also allows cardiologists to detect stenoses in addition to a culprit lesion in patients with myocardial infarction. Recent studies have indicated that lesions with a smaller degree of stenosis are actually the most likely to cause an acute coronary syndrome. As discussed by Dr. R.L. Wilensky of the Hospital of the University of Pennsylvania (Philadelphia) at the ESC congress, results from one study indicate that 5.8% of patients who have a percutaneous coronary intervention require a new intervention at a different lesion, or suffer a heart attack or acute coronary syndrome, within one year. The rate at which patients have such events is proportional to the degree of atherosclerosis (one, two or three vessel disease).
At present, the only means to predict the risk of a second event is the presence of multi-vessel disease and a prior PCI, but the accuracy of such predictions is not high. A similar finding is revealed by data from clinical trials with second-generation coronary stents. Those trials have shown that patients treated with drug-eluting stents are more likely to have a second event in a non-target vessel than in the initial target vessel. Up to 12% of patients who have had a PCI performed subsequently require non-target lesion PCI.
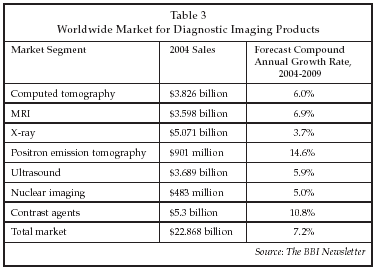
Now, CT angiography can be used to detect additional suspect lesions at the time of initial diagnosis, allowing them to be treated in the same encounter, preventing a second heart attack in a significant percentage of cases. According to GE Healthcare, there are 1,400 cardiology clinics that are prime candidates for implementation of 64-slice CT angiography. As shown in Table 3, the worldwide market for CT imaging equipment is estimated at $3.8 billion in 2004, with growth forecast at 6% over the next five years.
CT imaging also is playing a role in enhancing the capabilities of cardiologists in the angiography suite. The development of advanced flat-panel detectors has enabled the implementation of 3-D CT angiography using biplane image acquisition. Using equipment such as the Axiom Artis from Siemens, a 3-D image of the coronary arteries can be constructed in the cath lab, overcoming the limitations of 2-D imaging in assessment of coronary lesions. 3-D imaging allows detection of certain lesions that are not apparent on a 2-D image, and also allows more exact sizing of a lesion for stent selection. At present, the main limitation of 3-D CT angiography is in imaging of regions containing large amounts of calcified plaque. While the presence of calcification is one indicator of coronary artery disease, some calcified plaques may in fact be stable regions without a significant stenosis. Evaluation of such lesions requires use of invasive techniques.
An even more powerful approach to characterization of coronary lesions, described at the ESC congress by H.R. Schelbert of the David Geffen School of Medicine at the University of California at Los Angeles, involves the combined use of CT and radiolabeled PET imaging. Schelbert described the use of Annexin V, a targeted imaging agent from North American Scientific (Chatsworth, California) labeled with a variety of isotopes, to obtain PET images of plaque. Those images can be fused with non-invasive CT angiography images to allow evaluation of plaque in the carotid arteries. 18F-FDG is another promising agent for use in PET imaging of plaque. Such fusion images can allow identification of vulnerable plaque that is at risk of rupture, since they not only reveal flow-limiting stenosis but also detect plaque metabolic activity.
Remote monitoring of patients with cardiovascular disease was discussed in a number of sessions at the ESC congress. Monitoring of patients in the home, particularly patients with congestive heart failure (CHF), has developed into a significant market in the U.S., but is just beginning to emerge as a market in Europe. The driver of the market is reduction of healthcare costs by moving care into the community in order to reduce the utilization of more expensive hospital-based care.
As discussed by Professor Michael Oeff of Stad-tisches Klinikum Brandenburg (Germany), management of patients with congestive heart failure is a key application for telemonitoring in Europe. Oeff des-cribed a study involving 40 CHF patients monitored with the Vitaguard multiparameter telemonitoring sys- tem manufactured by Getemed (Teltow, Germany). The Vitaguard system monitored body weight, blood pressure, ECG, respiration rate and oxygen saturation, and also allowed patients to input clinical symptoms such as shortness of breath and data on drug use. Data from each patient was uploaded to a telemonitoring center daily, and reviewed by a nurse. If the data indicated a patient required intervention, a physician was contacted to provide the appropriate orders.
Hospital admissions and days of hospital stay were tracked for one year prior to implementation of monitoring, and for the year during which the patients were monitored. The number of admissions dropped from 39 to 15, while the days of hospital stay dropped from 478 to 176. The use of multi-parameter monitoring provided a clearer indication of patient condition and of the type of intervention required compared to monitoring of body weight alone. The cost of monitoring ranged from EUR 2,000 to EUR 3000 per year, vs. a cost of EUR 300 per day for hospitalization. Insurance companies in Germany are now providing reimbursement for the equipment and monitoring service.
Companies exhibiting home and alternate-site monitoring products at the ESC congress included Microlife AG (Heerbrug, Switzerland), with home blood pressure monitoring equipment; DailyCare Biomedical (Chungli, Taiwan), with home ECG monitors; Ortivus AB (T by, Sweden), a company supplying wireless 12-lead ECG monitors used in ambulances, hospitals, and the home; Equipmed (Gladesville, Australia), with wireless multiparameter monitors for in-hospital and home telemonitoring, and Cardiette (Milan, Italy), with an at-home single or 12-lead ECG system.
In addition, Philips Medical Systems (Andover, Massachusetts) exhibited the Motiva monitoring system, a television-based device that monitors blood pressure, ECG, blood glucose, oxygen saturation and body weight, and also provides interactive communication between a telemonitoring center and a patient in the home to relay medication reminders, alarms, and other health-related messages. The system uses a broadband network connection for communication between a telemonitoring center and a home-based central station. At present, a separate modem-based system is used to transmit vital signs data, but a new version now under development will incorporate vital signs data into the broadband system. The home-based station is connected to various home telemonitoring devices via a wireless connection.
An evaluation of the system in a 630-patient study is being conducted by Philips and Achmea Zorg (Zeist, the Netherlands), one of the largest health insurers in that country. A usability study also is being conducted by Philips in the U.S. in collaboration with Comcast (Philadelphia). In Europe, the Motiva system is leased to healthcare providers at a rate of EUR 150 a month. A prior study with the Philips home telemonitoring system involving a total of 418 patients, the Trans-European Network-Home-Care Management System (TEN-HMS) Study, found that heart failure mortality was reduced through the use of telemonitoring as compared to usual care or nurse telephonic monitoring.
