BBI Contributing Writer
SAN FRANCISCO, California — Minimally invasive breast disease management — including surgical and biopsy techniques as well as partial breast radiation therapy following surgery — continues to evolve at a rapid pace, in part due to the increased awareness and demand by knowledgeable patients. This consumer demand for a more cosmetic result, but with equivalent efficacy, has been fueled by the publication of studies and clinical trials available on the Internet. These driving forces, coupled with new technologies, have led to new products serving breast cancer patients; many of which were demonstrated at this year's American College of Surgeons (Chicago, Illinois) annual meeting in the Moscone Convention Center in October.
Percutaneous biopsy products
Although open surgical biopsy has been the standard of care for obtaining the best diagnostic measurement of carcinoma within breast tissue, percutaneous (or minimally invasive) image-guided breast biopsies are less invasive, less traumatic, less disfiguring, and less costly. For these reasons, percutaneous biopsy is becoming the preferred surgical procedure for most patients with image-detected abnormalities. Industry estimates are that about 60% of all percutaneous biopsies are being performed on both U.S. coasts, as well as in the Chicago area. In more rural areas, open surgical biopsies are still prevalent. However, recent graduates in breast surgery programs prefer percutaneous technologies. These trends suggest that minimally invasive breast biopsies will replace most open surgical biopsies, so it was not surprising to see several companies addressing this opportunity by showing their new percutaneous devices on the exhibit floor (Table 3).
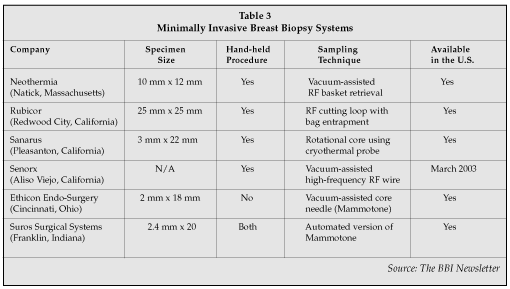
Sanarus Medical's (Pleasanton, California) biopsy system utilizes a quick "stick freeze" to immobilize the lesion and the cutting cannula rotates over the needle and through the lesion to provide a large, reliable tissue sample. It removes several samples and is attractively priced at $5,000 — well below other biopsy systems intended for office use that usually range around $20,000. It continues to sell their original cryoablation unit for ablation of fibroadenomas (benign tumors), which account for up to 60% of all biopsies. In the past, fibroadenomas, once diagnosed, were left in the breast, but lately more patients are demanding that their fibroadenomas be removed for their own peace of mind.
A new product from Senorx is focused on expanding the core needle biopsy marking marketplace; addressing the relatively new trend for surgeons to manage the patient following a suspicious finding on screening mammogram — often in their office. According to Lloyd Malchow, CEO of Senorx, "We see an under-appreciated, robust market opportunity in core needle biopsy marking systems. The introduction of the core needle biopsy marking system now allows us to pursue 500,000 annual core needle cases globally."
Rubicor (Redwood City, California) plans on beginning its clinical trials in the U.S. this month, with a proposed market launch in February 2003. The unique feature of its product is that it isolates and extracts the tissue by wrapping a bag around the lesion as it excises with its RF wire loop.
Minimally invasive lumpectomy products
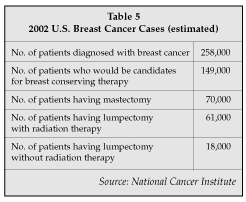
Not until October was the final word received regarding long-term efficacy comparing lumpectomy to mastectomy. In two different studies, both published in the New England Journal of Medicine, researchers from Italy and the U.S. concluded that long term outcomes for women who have a mastectomy are the same as for those who have a lumpectomy. Now that these studies, which followed 2,500 patients for 20 years, are complete, it is expected that all of the percutaneous biopsy companies (Table 4) also will develop a modified product — or use their biopsy device, if appropriate, for performing lumpectomy as well.
With the movement toward minimally invasive lumpectomies comes an increased requirement for precision localization of the lesion. Senorx displayed its lesion localization device that was touted in the October issue of Journal of Surgery as having equivalent safety and efficacy compared to standard localization wires, but with improved effectiveness. Dr. Philip Israel, director of the Breast Center (Marietta, Georgia), authored the paper that found fewer positive margins when using the new localization system than when using a standard system.
Breast tissue ablation
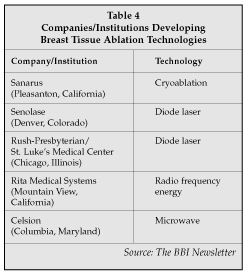
Nonsurgical ablation of breast tissue is currently questionable in the eyes of most breast surgeons. However, the evolution of minimally invasive techniques to remove tissue with the least amount of cosmetic impact, leads to the inevitable deduction that it may be possible to remove the offending tissue without any incision at all. Several companies are already addressing this potential opportunity by developing products that can ablate a lesion (most likely benign lesions at first) using an energy source such as laser, radiofrequency, microwave or cryoablation (Table 5).
Monica Morrow, MD, of Northwestern University (Evanston, Illinois) said she believes that the rationale of such techniques is somewhat questionable, as traditional surgical removal of a primary breast cancer is now a brief procedure with low morbidity and rare mortality. Having said this, she noted that nonsurgical ablative techniques have been carried out in a small series of patients; best for those with a single tumor measuring less than 3 cm, and in whom the tumors are more than 1 cm from the skin surface. In a series of 26 such patients, complete tumor necrosis was seen in 25 of the 26 (96%), without serious toxicity. Morrow also said that the fat necrosis that results after such tumor ablation is clinically and radiologically indistinguishable from the tumor itself and makes long-term follow up of these patients very difficult. Overall, she believes that with the excellent efficacy and acceptability of standard surgery for breast-conserving treatment, the newer ablative techniques remain mainly a curiosity or an alternative for patients who are totally opposed to standard surgical techniques.
Dr. Eva Singletary, professor of surgery at the University of Texas MD Anderson Cancer Center (Houston,Texas), has used the Rita Medical Systems (Mountain View, California) RF ablation system on 17 patients and found 16 of the tumors totally eradicated. One patient was found to have tumor cells elsewhere in the breast that had not been seen initially. Singletary is slated to begin another study of 30 patients this spring and said she feels that with good long-term results, ablation may be the surgery of choice for about 25% of all breast cancer patients.
Senolase (Denver, Colorado) uses a diode laser to ablate breast tissue. Although it was not exhibiting at ACS, the company is scheduled to start clinical studies at St. Luke's Hospital (Kansas City, Missouri) as well as with Dr. Steven Harms of the University of Arkansas for Medical Sciences (Little Rock, Arkansas). Dr. Suzanne Klimberg of the latter institution chaired a session on breast disease at the ACS conference and also is performing a clinical trial randomizing laser or RF ablation in order to determine clinical outcomes as well as patient comfort. She said she feels that if the ablative techniques cause enough pain to require general anesthesia in an operating room, then no ground has been gained in the quest for an effective office procedure. Klimberg said she is concerned that the amount of RF energy required to ablate tissue as opposed to just cutting it may cause too much discomfort to the patient, requiring general anesthesia in an operating room. She also voiced concern in that ablative techniques do not provide clean margins, which offers both the patient and surgeon some degree of comfort knowing that "they got it all."
Partial breast radiation
It may be said that lumpectomy is to mastectomy as partial breast radiation is to total breast radiation. That is, when a surgeon performs a lumpectomy, he feels that all the cancer is able to be removed without a complete mastectomy. Likewise, thought leaders in radiation therapy suggest that radiation therapy applied to a 2 cm depth of the lumpectomy bed is as effective in killing potential remaining cancer cells as radiating the whole breast. Current standard of care following surgical excision of breast cancer for primary single lesion cancer is external beam radiation to the excisional bed for 16 to 30 days following surgery. This is the same for both lumpectomy and mastectomy patients.
In fact, in the October issue of the New England Journal of Medicine, Dr. Bernard Fisher of the University of Pittsburgh (Pittsburgh, Pennsylvania) reported similar outcomes for both lumpectomy and mastectomy patients using the same radiation program following surgery. Partial breast irradiation or breast conserving therapy (BCT) would eliminate the side effects of whole breast irradiation, such as patient discomfort for several months following treatment and potential tissue erosions 10 to 20 years later. Not all breast cancer patients are candidates for BCT. Only those that have a single, primary tumor less than 2cm in size and without sentinel node involvement would currently be considered for it (see Table 5).
Breast-conserving therapies emerge
The finding that partial breast irradiation provides equal protection as whole breast irradiation has allowed new breast conserving therapies to emerge (Table 6). Intensity modulated radiation therapy (IMRT) delivers a focused eternal beam X-ray into a defined target without a catheter. IMRT can be administered in about 15-minute sessions over 10 to 16 days (vs. the 33-day treatments with standard external beam therapy) on an outpatient basis. Varian Medical Systems (Palo Alto, California) has developed software to deliver IMRT that can be added to existing external beam devices (that sell for $1 to $2 million) for around $100,000 to $200,000. IMRT can be used for other cancers as well, including prostate cancer and benign prostatic hyperplasia, so the hospital can share the IMRT with other specialties. New reimbursement codes for IMRT allow for a very profitable return on investment for the hospital.
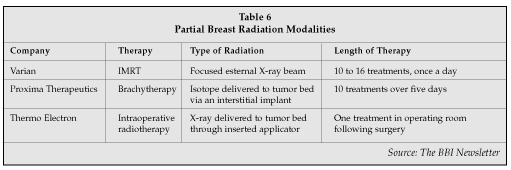
Proxima Therapeutics (Sunnyvale, California) is selling a brachytherapy device which delivers local radiation to the lumpectomy cavity. A balloon is inserted at the time of surgery into the lumpectomy cavity and is pumped up so that it touches all the walls of the cavity. An isotope is then placed into the balloon twice a day, for 10-minute treatments, over five days following surgery. The main advantage is that the patient has completed therapy in only five days, and that only local tissue is involved without any unnecessary radiation to surrounding tissue. Conceptually simple and elegant, in reality it precludes the patient from doing much else for those five days of treatment, in part due to external catheter protrusions prohibiting the patient from wearing normal clothing. Although reimbursement codes are in place for brachytherapy, bottom-line profit contributions to the hospital still favor IMRT. This is anticipated to equal out over time. Brachytherapy has been well received, with Proxima gaining approximately 10% market share of appropriate patients in only its first year following FDA approval. There is some skepticism as to whether continued market growth will occur until a second-generation device has worked out some of the issues mentioned above.
An attempt at achieving complete radiation efficacy in a single localized dose intraoperatively is being studied by Thermo Electron (Waltham, Massachusetts). It has developed and is in clinical trials with a linear accelerator that delivers X-rays through a percutaneous catheter to the lumpectomy cavity intraoperatively. It also can deliver to an open lumpectomy site, if that is the surgeon's preference. This is performed in the operating room with a single treatment that lasts from 30 to 60 minutes. The advantages are that it is a single treatment provided at the time of surgery and does not require subsequent outpatient treatments, which is especially advantageous for patients who do not live nearby. The disadvantage is that it requires a large, expensive ($400,000) piece of equipment in the operating room. The idea of a linear accelerator in the OR is one that is catching on in other sub-specialties such as neurosurgery, and has the potential to be cost shared by several surgical specialties as more intraoperative radiation treatments are adopted.
The successful BCT product will be the one that can deliver the necessary radiation in the fewest amounts of treatments with the least side effects, and at the overall lowest cost to the hospital. This is true provided that the newer treatment provides the same long-term efficacy as that of full or partial breast radiation. This cannot be fully determined for at least 10 years, but market adoption of new technologies has often been shown to occur after only five years of data, frequently when the data is a logical, follow-on to current therapies. Only three-year data is available on most of these therapies, but the results are promising. So far, patients and surgeons have demonstrated a willingness to accept some small amount of lesser efficacy in return for a better cosmetic effect.
