CDU Contributing Editor
WASHINGTON – Risk assessment for cardiovascular disease is a rapidly evolving field, encompassing in vitro diagnostics as well as imaging modalities and physiological measurements. The demand for more powerful risk assessment techniques is being driven by major advances in medical and interventional therapy. Early detection of cardiovascular disease now allows therapies to be implemented that can significantly reduce the incidence of myocardial infarction (MI) and related deaths. Furthermore, continued improvements in cardiovascular disease therapies such as advances in coronary stent technology are making them more durable, prolonging disease-free intervals and increasing the value of risk assessment tools.
Although the treatment of cardiovascular disease is improving, its incidence is continuing to grow. As shown in Table 1, total cardiovascular interventions and surgical procedures are rising, as is the incidence of serious hypertension, congestive heart failure, and stroke. In addition to aging of the population, other factors responsible for the trend include increased prevalence of diabetes and obesity in the U.S. population, and the impact of improved therapies that, while improving survival in myocardial infarction patients, result in growth in the number of patients who go on to develop heart failure. Other factors that are attracting increased attention for their role in cardiovascular disease include inflammatory conditions and genetics. In particular, genetic testing, both to identify individuals with known cardiovascular disease genes as well as to detect genetic determinants that may predispose an individual to develop disease in combination with other risk factors, represents an area of considerable research investment.
 |
The number of tests used to assess risk is expanding. Total cholesterol assays, once used as the sole blood marker for risk assessment, are now accompanied by a panel of lipoprotein assays, and new markers such as high-sensitivity C-reactive protein (hs-CRP), tests of coagulation system function, and heart failure markers are becoming part of the battery of tests used. New applications are emerging for existing tests such as troponin markers, while additional tests to further improve early detection of myocardial infarction are in development. Advances in imaging technologies such as electron beam computed tomography, magnetic resonance imaging and cardiac ultrasound also are improving the ability of physicians to detect cardiovascular disease at an early stage, resulting in additional demand for follow-up in vitro testing. The evolution of cardiac risk assessment technologies was the topic of the 25th annual Beckman Conference held here last spring, sponsored by the American Association for Clinical Chemistry (AACC; Washington) and including presentations by some of the leading experts in the field.
Genetic testing to play larger role
Genetic testing to detect inherited cardiovascular disease can now detect a considerable range of genomic risk factors. As discussed by Christine Seidman, MD, of Harvard Medical School (Boston, Massachusetts), gene detects have now been identified that are responsible for 20% to 25% of idiopathic dilated cardiomyopathy. Advances in echocardiography using equipment from suppliers such as Philips Medical Systems (Best, the Netherlands), Toshiba Medical Systems (Tokyo), GE Medical Systems (Waukesha, Wisconsin) and Siemens Medical Solutions (Munich, Germany) have revolutionized the ability to detect cardiomyopathy at an early stage. Using that technique, researchers have been able to detect relatives of cardiomyopathy patients who have both a detective gene pattern and early-stage, asymptomatic disease. Seidman said that clinicians are now obligated to test close relatives of cardiomyopathy patients for the associated genetic defects, since those individuals could then be managed to mitigate the long-term effects of their condition, which is a leading cause of congestive heart failure (CHF). Furthermore, the genetic abnormalities, which affect 10 different genes, are very heterogeneous. Some mutation patterns lead to hypertrophic cardiomyopathy, while others do not, perhaps because of a protective factor that is dominant in some cases, or because of a second-hit phenomenon that occurs only in some individuals. Seidman said she believes that DNA sequencing to detect cardiovascular disease genes and analyze risk profiles will become clinically important, perhaps in the very near future.
As discussed at the Beckman conference, by Edward Rubin, MD, PhD, of Lawrence Berkeley National Laboratory (Berkeley, California), another gene defect identified as Apo A5 is associated with triglyceride levels. Although a direct correlation of the Apo A5 gene pattern with cardiovascular disease has not yet been shown, the relationship of triglyceride levels with disease is well-known. Although testing of triglyceride levels is obviously a simpler and more direct approach to detecting such a condition, choice of the treatment that will prove most effective in reducing long-term risk may be strongly influenced by the underlying genetic cause. Genetic testing for cardiovascular disease already is available for a number of markers. For example, Myriad Genetics (Salt Lake City, Utah) offers the CardiaRisk test used to assess cardiovascular disease risk in hypertensive patients. The $295 test detects presence of the T235 variant of the angiotensinogen (AGT) gene. Myriad also has reported the discovery of a gene linked to early-onset coronary heart disease, called CHD2. Other genetic markers associated with cardiovascular disease risk include Factor VIII and Factor IX Deficiency markers and markers of Factor V Leiden Syndrome.
As shown in Table 2, the genetic testing services market is expanding rapidly, and further growth is expected as additional diseases including other cardiovascular conditions become amenable to diagnosis via genetic testing. The market for DNA-based genetic testing services in the U.S. is expected to grow from $167 million in 2002 to $372 million by 2007, representing a compound annual growth rate of 17%. Given the high prevalence of cardiovascular disease, testing for genes associated with such diseases is likely to comprise a sizeable proportion of the products and services market in the future.
 |
Emerging CVD risk factors
Many of the key risk factors for cardiovascular disease (CVD), such as hypertension, obesity, a diet high in saturated fat, age and male gender are widely recognized. However, the importance of certain other factors such as the levels of various lipoprotein markers, levels of oxidized amino acids and inflammatory markers, and diabetes has only recently become apparent. Diabetes is a particularly important factor because of its strong impact on cardiovascular disease risk, the significantly poorer prognosis for patients with cardiovascular disease who also are diabetics, and the growing prevalence of diabetes worldwide. Type 2 diabetes is a particularly concern because at least one-third of all individuals with the disease are unaware that they have it. In addition, as discussed by Jerry Nadler, MD, of the University of Virginia (Charlottesville, Virginia), elevations in average blood glucose to between 140 and 200 mg/dL are sufficient to predispose an individual to cardiovascular disease even though those levels do not meet the current criteria for classification of an individual as a diabetic.
The worldwide prevalence of Type 2 diabetes is projected to increased from 151 million in 2000 to 221 million by 2010, according to studies cited by Nadler, corresponding to an annual growth rate of almost 4%. A factor of particular concern in the U.S. is the growing prevalence of diabetes among the younger population. Between 1990 and 1998, the prevalence of diagnosed diabetes increased 70% in the 30-39 year-old age group, vs. an increase of 31% in the 50-59 age group and a 33% increase in the total population. One factor responsible for that trend may be an increase in visceral obesity in the younger age group. Whatever the cause, the prolonged exposure to high glucose levels characteristic of diabetes is likely to increase the risk of developing cardiovascular disease, as indicated by the fact that more than 80% of all deaths among Type 2 diabetics are attributable to cardiovascular disorders such as coronary atherosclerosis, cerebrovascular disease or peripheral vascular disease.
Those statistics underlie the growing emphasis on improving techniques to screen for diabetes and on more frequent glucose testing to attain tighter control of glucose levels in diagnosed diabetics, since studies have demonstrated that early detection of the disease, coupled with subsequent diet and lifestyle modifications to normalize blood glucose levels, can have a significant impact in reducing atherosclerosis. New techniques under development for diabetes screening include HLA gene analysis for detecting Type 1 diabetes as well as the Accu-Chek D-Tector from SpectRx (Norcross, Georgia) and Roche Diagnostics (Mannheim, Germany), a noninvasive optical device now scheduled for launch in Europe in 1Q03. Another emerging test that may prove important in managing patients with early-stage diabetes to help minimize long-term complications is intracellular magnesium. A number of studies cited by Nadler have indicated that magnesium levels may play an important role in protecting against the effects of diabetes, and perhaps in preventing the onset of disease. At present, the best available technique to measure intracellular magnesium is nuclear magnetic resonance (NMR) spectroscopy, a method that is not widely available, although companies such as LipoMed (Raleigh, North Carolina) use the technique to analyze lipid particle profiles as an indicator of the risk of heart disease. However, new intracellular sensor-based probes, using ISE technology, are under development that may address that issue, according to Nadler.
Another important area of research that may lead to the identification of new risk markers for cardiovascular disease is the study of lipoprotein processing pathways in the development of vascular degradation, and in particular the role of oxidized LDL. Various clinical trials, such as the Heart Outcomes Prevention Evaluation (HOPE) study, have assessed the ability of antioxidants to prevent the development of cardiovascular disease. Although some initial trials indicated that vitamin E could act as a protective agent, based on an 80% drop in non-fatal myocardial infarction reported in the CHAOS trial, subsequent larger trials such as HOPE did not show a beneficial effect. Similar studies using vitamin C have also failed to produce positive results. Nevertheless, as discussed by Jay Heinecke, MD, of Washington University School of Medicine (St. Louis, Missouri) at the Beckman gathering, oxidized amino acids are strongly associated with the development of vulnerable plaque, and have been found in ruptured plaques in human studies. Matrix metalloproteinase is another marker that is associated with the presence of oxidized products in ruptured plaques. The potential exists to develop in vivo imaging methods targeting oxidized products or perhaps matrix metalloproteinases as a method to identify vulnerable plaques.
In vitro testing products to monitor free oxygen radicals as markers of oxidative damage already are available in Europe, such as the FORM analyzer from Incomat Medizinische Gerate (Glasshutten, Germany). Studies conducted by Wojciech Wojakowski, MD, PhD, at the Silesian Medical Academy (Katowice, Poland) have shown that free radical damage can occur in children from families with early-onset heart disease as early as 6 years of age.
But most attention in the field is focused on the use of lipoprotein panels, inflammatory markers and markers of myocardial damage as the next generation of risk assessment tools. Data is now available on the diagnostic utility of various recognized risk markers that demonstrates the relative predictive power of individual tests as well as of combinations of tests in detecting patients at risk for cardiovascular disease.
As discussed by Nader Rifai, PhD, of Children's Hospital and Harvard Medical School (both Boston, Massachusetts), a combination of hs-CRP with the ratio of total cholesterol to HDL cholesterol is correlated with the highest relative risk for future myocardial infarction of any set of biochemical risk markers evaluated. That combination corresponds to a relative risk of 5.0 vs. a figure of less than 3 for the total cholesterol/HDL cholesterol ratio alone. In fact, the relative risk for an elevated hs-CRP level is slightly higher than for the total cholesterol/HDL cholesterol ratio, a parameter that is now reasonably well established as a risk assessment tool in cardiology. Total cholesterol alone, probably the most widely used risk marker, corresponds to a relative risk of about 1.5 when elevated, and is consequently a much less powerful predictor than the combination of hs-CRP and TC/HDL.
While some other analytes, including interleukin-6 and soluble ICAM-1, also show a high correlation with cardiovascular disease risk, they are unstable in vitro and are very difficult to measure reliably. On the other hand, at least 30 hs-CRP assays are now available worldwide, according to Rifai, and an even wider array of assays is available for measuring total cholesterol and HDL. Among the hs-CRP assays mentioned by Rifai as being particularly useful in risk assessment are tests from Diagnostic Products (Los Angeles, California), Iatron Laboratories (Tokyo), Wako Pure Chemical Industries (Osaka, Japan) and Denka-Seiken (Tokyo). All four tests have the sensitivity needed in order to accurately risk-stratify patients, whereas others that were evaluated gave significant overlap in classifying patients into risk quartiles.
Perhaps the most important recent finding comes from the Cholesterol And Recurrent Events (CARE) trial, which involved more than 4,000 patients who had already had a myocardial infarction and were subsequently treated with pravastatin. A much greater benefit was found if the patient had a high CRP level, indicating that statins such as pravastatin have an anti-inflammatory effect as well as a lipid-lowering effect. Furthermore, there was no association between the risk reduction due to CRP lowering and LDL lowering, indicating that two different mechanisms are responsible for the adverse events associated with the markers. A remaining issue with the use of CRP in routine clinical practice is the current lack of standardization of the various assays. However, as a result of a meeting earlier this year sponsored by the Centers for Disease Control and Prevention (Atlanta, Georgia), hs-CRP will soon be standardized and recommended as a standard part of a cardiac risk assessment panel. The test will not, however, be recommended for use as a general population screen, according to Rifai.
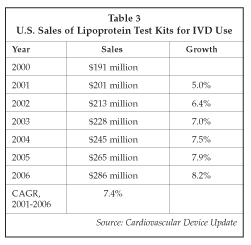
As a result of the anticipated increase in hs-CRP testing, as well as expanded use of various lipoprotein tests such as HDL cholesterol, the market for products used in cardiovascular disease risk assessment is expected to grow. Table 3 on page 4 presents estimates for sales of lipoprotein testing products for clinical use in the U.S. (excluding equipment), including test kits for total, HDL and LDL cholesterol as well as triglycerides. The market for CRP test kits is estimated at about $85 million in the U.S., but high-sensitivity tests account for only a small proportion of that total at present. In the future, however, the addition of hs-CRP to risk assessment panels should stimulate rapid growth in the market, particularly in light of the evidence that well-established treatments are now available for individuals with CRP elevations due to cardiovascular disease.
 |
Another set of factors that play a role in the risk of developing an acute coronary syndrome, stroke, or pulmonary embolism are the factors responsible for blood coagulation. Recent studies by Richard Becker, MD, of the University of Massachusetts (Amherst, Massachusetts) have shown that activation of platelets coincides with myocardial infarction, and studies of thrombosis markers have demonstrated that they can be used to predict the risk of death post-MI. Clearly, the tendency of the blood to clot under low or unstable flow conditions will have a major impact on the risk of occlusion in a narrowed coronary artery that undergoes plaque rupture. Perhaps most importantly from the standpoint of predicting future coronary events, elevations of coagulation markers have been observed in patients with defects in arterial blood flow, and could thus prove useful in assessing the likelihood of a thrombotic event. One marker studied extensively by Becker is P-selectin, a cell surface marker measured via flow cytometry that is correlated with platelet activation. A particularly intriguing finding is that P-selectin is a possible factor involved in the binding of platelets to leukocytes, perhaps providing a direct link between inflammation and thrombosis in acute coronary syndromes. Other markers of coagulation activity that become elevated in acute coronary syndromes include thrombin, fibrinopeptide A, and thrombin-antithrombin III (TAT).
The findings provide opportunities for development of new therapies to treat or perhaps help prevent myocardial infarction, as well as for tests to not only predict the risk of an event but also to help select the optimum drug for treatment. Already, a number of highly effective drugs including GPIIb/IIIa receptor antagonists such as ReoPro from Centocor/Johnson & Johnson (Malvern, Pennsylvania), clopidogrel, ticlopidine, tirofiban and eptifibatide have been developed that target various platelet receptors. Products for measurement of platelet activation or the degree of blocking of platelet receptors include the PFA-100 from Dade Behring (Deerfield, Illinois); the Ultegra from Radiometer Medical (Copenhagen, Denmark); the Clot Signature Analyzer from Xylum (Scarsdale, New York); and the Kowa AG-10, available in Japan from Kowa (Tokyo). The Afecta activated factor XII assay, from Axis-Shield Diagnostics (Dundee, UK), is another device that allows physicians to assess activation pathways in the coagulation system.
Troponin markers, used primarily in the initial diagnosis and triage of patients with chest pain at present, also appear to have applications in risk assessment, particularly for prognosis following therapeutic intervention. For example, as discussed by Allan Jaffe, MD, of the Mayo Clinic (Rochester, Minnesota), at the Beckman conference, recent evidence indicates that the success rate of primary angioplasty is correlated with elevation in troponin levels. Recently, troponin levels have been incorporated into the Braunwald classification scheme for coronary artery disease because major differences in prognosis have been found depending on the degree of troponin elevation. While suppliers of troponin assays have been aggressively investing to develop the more sensitive troponin assays needed for prognosis and risk assessment, Jaffe said that none of the available assays are yet sensitive enough. For example, about 11% of patients with angiographically evident coronary artery disease, including some who have acute coronary events, are troponin-negative. Such patients may have low-level ischemia that has not yet produced myocardial tissue damage, the latter being required in order for troponin to be released into the bloodstream.
Another potential application of troponin markers is identification of patients in the ICU with non-cardiac disease, such as COPD patients, who are suffering myocardial damage in the absence of detectable ischemia. Jaffe said that only about one-third of such patients are identified with existing monitoring methods. Other applications of troponin assays described by Jaffe include diagnosis of myocarditis, assessment of long-term cardiovascular disease risk in dialysis patients, highly sensitive detection of heart damage due to various drug therapies, and, possibly, prognosis in stroke patients related to concomitant heart disease. Suppliers of troponin assays for clinical use include Abbott Diagnostics (Abbott Park, Illinois), Bayer Diagnostics (Tarrytown, New York), Beckman Coulter (Fullerton, California), Biosite Diagnostics (San Diego, California), Dade Behring, Diagnostic Products, Roche Diagnostics, Ortho-Clinical Diagnostics (Raritan, New Jersey), Sigma Diagnostics/First Medical (St. Louis, Missouri) and Tosoh (Tokyo).
Markers for heart failure
Another segment of the cardiovascular disease testing products market that is expected to exhibit strong growth over the next few years is products to test for CHF. The segment has only emerged recently with the introduction of both point-of-care tests and, in Europe, tests for use in the central lab for the measurement of brain natriuretic peptide (BNP) and N-terminal pro-BNP, the two fragments of the pro-hormone proBNP that are released into the bloodstream after stimulation of cardiomyocytes by events such as myocardial stretch. The role of the heart in producing such hormones, i.e., in acting as an endocrine organ and not just as a mechanical pump, was proposed as early as 1964 according to John Burnett Jr., MD, of the Mayo Clinic, who discussed the topic at the Beckman conference. However, the growing magnitude of the public health problem posed by CHF has recently heightened awareness of the disease and stimulated the development of new diagnostic and therapeutic products.
As shown in Table 4, the prevalence of CHF is increasing rapidly. The increase is occurring in all age groups, indicating that it is not due solely to aging of the population. One contributing factor to increased CHF prevalence is improved survival for myocardial infarction patients as a result of better therapies. Many MI patients suffer heart damage that later progresses to CHF. Another recent finding is that half of the individuals with left ventricular dysfunction, the primary defect in CHF, are asymptomatic. Research aimed at developing an improved understanding of the etiology and progression of CHF is to be a focus of National Institutes of Health (Bethesda, Maryland) grant programs in the coming year, according to Burnett.
 |
Improved diagnostic tests for CHF are urgently needed, since studies cited by Burnett have shown that up to 50% of those with the disease may be incorrectly diagnosed in the primary care setting. Markers such as BNP and NT-proBNP represent a major advance in diagnosis and monitoring, since existing techniques such as echocardiography, radionuclide angiography and cardiac MRI are expensive and not widely available or not completely reliable in routine practice. Blood markers are expected to play a role both in improving primary diagnosis as well as in early detection of CHF, since activation of BNP is one of the initial events associated with the onset of left ventricular dysfunction. In addition, the recent introduction of new drugs such as Natrecor from Scios (Sunnyvale, California) for CHF treatment creates an opportunity to use the tests to monitor response to therapy, significantly expanding the market opportunity. Importantly, patients who are diagnosed early and treated with drugs such as ACE inhibitors have a definite benefit. However, patients are now not often treated aggressively once diagnosed because of a lack of reliable clinical indicators for assessing response. Studies using the new tests have shown a major improvement with the use of monitoring in CHF patients undergoing therapy, according to Burnett, although the studies were performed on a small, select group of patients. A larger follow-on study is planned. However, it is already evident from the data that BNP is superior to echocardiography as a monitoring tool.
Burnett recommends that BNP testing be performed on all patients admitted to the hospital for CHF as well as at discharge and on patients who have had a myocardial infarction. MI patients often suffer myocardial tissue damage that weakens the heart and progresses to heart failure, and early detection of that condition could allow more successful treatment. Burnett also said there should be serious consideration of the use of BNP as a screening tool in at-risk populations, since studies indicate there is a large population of individuals with early-stage CHF who are not identified. The Biosite BNP test was cleared for marketing in the U.S. in November 2000, and the company reported BNP sales of $1.8 million in 4Q01 vs. $63,000 in 4Q00. The test uses either whole blood or plasma samples. A total of 249 accounts were using the test as of year-end 2001. The test is priced at $29, excluding the cost for controls and calibrators, and the meter required to perform the test has a list price of $4,750. A test for NT-proBNP developed by Roche Diagnostics received a CE mark and was launched in Europe earlier this year. The test is in clinical trials in the U.S. The Roche test runs on the company's Elecsys system and can be performed using either plasma or serum samples. The assay time is 18 minutes, and the test requires only a 20-microliter sample.
In the future, the potential exists to use various genetic markers as an aid in CHF diagnosis. Activation of the Cardiotrophin-1 gene, which in turn drives synthesis of the cardio-fibroblast growth factor, is one key genomic trait. Cardiotrophin-1 activation precedes BNP activation in CHF, indicating that gene expression analysis could potentially be used to provide earlier detection of heart remodeling. Companies such as Roche Diagnostics that are now marketing immunoassay products for use in CHF management and that also have a strong technology base in genomic testing appear well positioned to benefit from future market trends. While sales of BNP products remain small at present, expanding physician adoption is likely in the future, particularly as primary care physicians become aware of the potential benefits of the tests. Testing products for CHF diagnosis and management have a clear potential to develop into a major new segment of the in vitro diagnostics market over the next few years.
