CD&D Contributing Editor
WASHINGTON — Cardiac markers, employed in diagnosis and prognosis for acute coronary syndromes both in central laboratories as well as at the point of care, have represented one of the most rapidly growing segments of the clinical diagnostics market over the past few years. The primary role at present for cardiac markers such as Troponin, creatine kinase-MB (CKMB) and myoglobin is in diagnosis of acute coronary syndromes, but there is growing interest in newer markers that can aid in assessing the risk for myocardial infarction, such as markers of unstable coronary plaque, as well as in stroke markers for initial diagnosis and risk assessment.
At the annual meeting of the American Association for Clinical Chemistry (AACC; Washington), held here in late July, leading experts in the field of cardiac markers described the latest developments, including advances in rapid diagnosis, unstable/vulnerable plaque detection, and integration of in vitro diagnostics and imaging in the management of cardiovascular disease. New developments in risk assessment, including assessment of stroke risk, also were described at the AACC conference, potentially expanding the scope of testing beyond patients with chest pain to the much larger population of individuals at risk for multiple types of cardiovascular disease.
Molecular diagnostics for cardiovascular disease is another emerging area that may enable more accurate identification of individuals in the general population who can benefit from comprehensive risk assessment, allowing preventative therapy to be initiated to avoid an acute event, as well as improved guidance of therapy.
The global market for cardiac markers is in excess of $1.1 billion, as shown in Table 1 About one-third of the market is attributable to sales of point-of-care cardiac marker testing products, with the remainder attributable to products for use in the central laboratory.
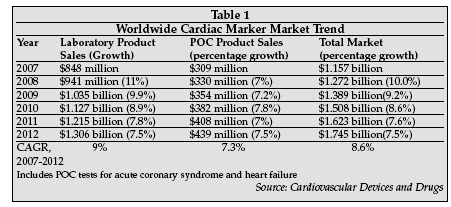
Leading suppliers of cardiac marker products include Beckman Coulter (Brea, California), Roche Diagnostics (Indianapolis, Indiana), Abbott Diagnostics (Abbott Park, Illinois), and Siemens Healthcare Diagnostics (Deerfield, Illinois) for central lab products; and Inverness Medical Innovations (Wal-tham, Massachusetts), Roche Diagnostics and Siemens Healthcare Diagnostics for point-of-care cardiac marker products.
At present, the market is dominated by sales of test kits for Troponin I and Troponin T, CK-MB, myoglobin, and brain natriuretic peptide (BNP and NT-proBNP). BNP and NT-proBNP are markers used both in diagnosis of patients with chest pain as well as in diagnosis and monitoring of heart failure.
Expanding role in disease management
One important area for new applications of biomarkers in cardiovascular disease is stroke diagnosis and risk assessment. According to the American Heart Association (Dallas) and the World Health Organization (Geneva, Switzerland), 780,000 strokes occur annually in the U.S. and 21 million occur worldwide. In addition, there are many more individuals who have survived a stroke and 30% to 50% live with varying degrees of disability. In the U.S., the National Institutes of Health estimates that there are 5.5 million stroke survivors. The cost of caring for disabled stroke survivors and other costs associated with stroke are estimated at $57.9 billion in 2006 in the U.S. There are currently no widely accepted biomarkers for stroke, making diagnosis reliant upon imaging methods such as MR, CT and PET scans, clinical symptoms, and cerebral angiography. Stroke is increasingly being managed much like a heart attack, with a high priority on restoring blood flow to the affected areas of the brain as rapidly as possible in the case of an ischemic stroke, and on stopping bleeding in the brain in the case of hemorrhagic stroke. However, the lack of a marker or panel of markers for stroke, as now exists for heart attack, limits the ability of clinicians to respond rapidly when a patient with a suspected stroke presents. The first line of treatment for occlusive stroke is intravenous infusion of tissue plasminogen activator (tPA), but there is a risk of inducing a hemorrhage with thrombolytic therapy, so an accurate diagnosis of stroke, including differentiation of ischemic versus hemorrhagic stroke, is essential prior to initiating treatment.
A new marker for stroke, and one that enables differentiation between the two different types of stroke, was described at the AACC conference by CIS Biotech (Atlanta). The CIS test detects the NR2 peptide fragment of the N-methyl-D-aspartate (NMDA) receptor, and is configured as a magnetic particle microplate enzyme immunoassay. Within three hours after a cerebrovascular event, NR2 is released and crosses the blood/brain barrier, becoming detectable in venous blood. The marker persists in blood for several days after an event.
NR2 is mainly released in ischemic stroke, and not due to cerebral hemorrhage, allowing clinicians to differentiate between the two types of stroke. The test has been licensed by Siemens for applications in clinical diagnostics, and an 800-patient clinical trial is in progress at four hospitals in the U.S. to validate the utility of NR2. Clinical studies have also been performed in Germany, Russia, Romania and the UK. The marker can detect strokes that are greater than 3 ml in volume, and the marker level is proportional to stroke size.
Another test being developed by CIS Biotech measures antibodies generated in response to NR2 in affected patients. Since NR2 normally is not present in the blood, the immune system responds as it would to a foreign substance, and generates antibodies. The presence of NR2 autoantibodies is thus indicative of a stroke or a transient ischemic attack (TIA) that occurred previously. The delay between an event and the appearance of detectable antibody is between three days and 3 to 6 months. Consequently, the NR2 antibody test has potential utility in identifying patients who have had a TIA or a small, unrecognized stroke and are at risk for a major stroke, enabling treatment with preventative therapy such as anti-coagulation or interventional methods.
Another test for stroke risk was exhibited at the conference by diaDexus (South San Francisco, California). The PLAC Test measures serum levels of lipoprotein-associated phospholipase A2 (Lp-PLA2), a marker that has been shown to be associated with the presence of rupture-prone plaque. Lp-PLA2 also has applications in assessment of heart attack risk. The PLAC test is FDA-cleared, and received a CPT code assignment in January of 2007, enabling labs to obtain reimbursement for the test.
diaDexus is marketing the PLAC test through multiple clinical reference laboratories in the U.S., and has experienced a doubling in volume of PLAC tests performed each year since the test was introduced in 2003. The company has recently developed a turbidimetric immunoassay version of the PLAC test which can be performed on various automated instruments such as the Modular P analyzer from Roche Diagnostics and chemistry analyzers from Olympus (Tokyo).
Many experts in stroke diagnosis and risk assessment believe that a panel of markers will be needed in order to achieve clinically acceptable sensitivity and specificity for stroke diagnosis. Biosite (San Diego), now a unit of Inverness Medical Innovations, has evaluated a prototype stroke marker panel consisting of six biomarkers (S-100b, B-type neurotrophic growth factor, von Willebrand factor, matrix metalloproteinase-9, and monocyte chemotactic protein-1), but withdrew its PMA submission for the test.
Randox Laboratories (Antrim, UK) exhibited two test panels designed for applications in neurological diagnosis, the Cerebral Array I and II, at AACC. The test panels, currently available for research use only, are performed using the Randox biochip array technology and the company's Evidence and Evidence Investigator analyzers. Up to seven analytes can be measured simultaneously, and sample volumes as low as 25 uL can be analyzed.
The Cerebral Array 1 panel includes four markers (brain-derived neurotrophic factor or BDNF, h-FABP, GFAP, and IL-6), while the Cerebral Array II includes seven markers (NSE, NGAL, sTNFR, von Willebrand Factor, D-dimer, thrombomodulin, and CRP). The markers are in most cases not specific for cerebral tissue, but instead are general markers of cardiovascular status.
Vulnerable plaque a large opportunity
Perhaps the most significant new market opportunity for cardiac markers is in detection of vulnerable or unstable plaque. Plaque rupture and the ensuing thrombosis which occurs at the site of rupture is the leading cause of myocardial infarction, and is also responsible for 87% of strokes. A marker or marker panel that could accurately detect vulnerable plaque in advance of rupture and enable preventative therapy to be implemented to avoid a heart attack or stroke would address a major unmet clinical need, and a significant market opportunity.
At the AACC conference, a symposium on biochemical markers of unstable coronary plaque addressed the latest developments in biomarkers, including new markers under investigation for early detection of vulnerable plaque as well as the possible role of existing markers as an aid to vulnerable plaque detection and characterization. Alan Wu, PhD, of San Francisco General Hospital, described a number of markers that are being studied for detection of unstable coronary plaque, including myeloperoxidase (MPO), pregnancy-associated plasma protein-A (PAPP-A), Placental Growth Factor (PlGF), Monocyte Chemoattractant Protein-1 (MCP-1), Ischemia-Modified Albumin (IMA), and Plasminogen Activator Inhibitor-1 (PAI-1).
Wu emphasized that any new marker must meet certain requirements in order to be successful. First, it must add incremental value to existing markers. In addition, it must have a link to therapy, either in treatment selection or monitoring of response. The pathophysiologic role of the marker also should be understood, as should any genetic links of the marker.
While all of the candidate markers listed by Wu have shown some correlation with factors such as risk of death and myocardial infarction, atherosclerosis development, or response to statin therapy, no single marker has emerged as a definitive identifier of vulnerable plaque. That has led Wu to investigate multimarker strategies for early detection of acute coronary syndromes, combining results from a number of markers each of which indicates some aspect of unstable plaque to create an index that predicts the risk of an event. Such multimarker tests have already been introduced for cancer testing, such as the MammaPrint test used in breast cancer management.
The FDA created a new category of diagnostic products, In-Vitro Diagnostic Multivariate Index Assays, or IVDMIAs, in 2006 that applies to such tests. So far, no IVDMIAs have been introduced for cardiovascular diagnostics, although a number of suppliers are developing individual markers that may become components of an IVDMIA. For example, the Biosite unit of Inverness Medical Innovations has licensed rights to MPO from the Cleveland Clinic Foundation, and Abbott Diagnostics is developing an MPO assay for its ARCHITECT immunoassay platform. Beckman Coulter is developing assays for PAPP-A and PlGF for its immunoassay platforms.
While obtaining regulatory clearance for those tests as individual markers may prove straightforward, Wu anticipates a number of challenges in introducing panels of vulnerable plaque markers in an IVDMIA format. Challenges include reimbursement issues, since multimarker tests are likely to be expensive, as well as the complexity of performing such tests in the lab. Complexities arise in performing quality control checks and proficiency testing for multiple markers measured simultaneously. IVDMIAs also generally require use of bioinformatics to produce an actionable result, combining the data from the individual markers using a score-generation algorithm, neural network, regression tree, or other methodology to produce an index or classification which is reported to the physician.
Questions arise as to whether only an index should be reported, or whether individual results for each marker in the panel should be provided to the clinician. Based on the recently revised guidance from the FDA on IVDMIAs, available on the FDA's website, a multimarker panel test may not be classified as an IVDMIA if results for each marker are reported and the analysis of the combined results to produce an index is a procedure that the physician could perform independently.
Another potential marker for vulnerable plaque, discussed by Oliver Danne, MD, of Charite Universitatsmedizin (Berlin, Germany), at the AACC symposium, is whole-blood choline. Whole-blood choline levels are closely related to blood cell choline levels, whereas plasma choline is a relatively short-lived marker for ischemia, with a half-life of 10 minutes. The short half-life of plasma choline makes it difficult to use as a marker of vascular ischemia. Blood cell choline levels, on the other hand, have been shown to be related to the occurrence of plaque rupture, and may also be an indicator of the type of acute coronary syndrome that is present.
Levels of whole-blood choline show a graded increase from unstable angina to ST segment elevation MI with left bundle branch block, increasing further in non-ST segment elevation MI, and are highest for an acute coronary event with arrhythmia and need for cardiopulmonary resuscitation.
Choline may also play a role in the emerging integration of in vitro diagnostics and imaging. A fluorinated derivative of choline, 18F-F choline, has been found to be a better identifier of vulnerable plaque in animal studies than 18F-fluorodeoxyglucose, the most commonly used imaging agent in PET/CT studies. Consequently, the possibility exists for use of in-vitro diagnostic whole-blood choline testing as a screen for the presence of vulnerable plaque, followed by imaging with 18F-F choline to localize the lesion so it can be treated.
Allan Jaffe, MD, of the Mayo Clinic (Rochester, Minnesota), another presenter at the AACC symposium on unstable plaque, described recent findings with existing cardiac markers such as Troponin I that indicate they may also have a role in identifying patients with vulnerable plaque who are at increased risk for a subsequent event. Newer high-sensitivity troponin assays, such as a research-use assay from Singulex (Alameda, California) for cardiac Troponin I which can measure troponin levels down to 0.8 pg/mL with 10% precision, have allowed researchers to study the correlation of low levels of troponin, a marker of cardiac tissue damage, with the rate of adverse coronary events in patients who have had an infarction.
The Singulex assay employs nanofluid single molecule fluorescence detection, and is performed using the company's Erenna system. Sensitivity of the Erenna troponin assay is about 100-fold higher than that of first-generation troponin I tests.
Other high-sensitivity troponin tests include the TnI-Ultra assay available on the Advia Centaur immunoassay system from Siemens Healthcare Diagnostics, with a sensitivity of 30 pg/mL for 10% precision; the Stratus CS Acute Care TnI, with a sensitivity of 60 pg/mL, also from Siemens; and a new high-sensitivity troponin assay from Beckman Coulter, the AccuTnI assay.
Studies performed by Jaffe using high-sensitivity troponin tests have shown that the marker has better predictive accuracy for future coronary events than certain other risk prediction markers such as GPBB and hFABP. One study published by Jaffe in 2007 which analyzed 448 patients who presented with chest discomfort over an eight-year interval following their initial event found that those with a TnI level above 20 pg/mL had a significantly higher event rate for MI and heart failure compared to those with levels below 20 pg/mL. Jaffe said he believes that other markers of vulnerable plaque should be re-evaluated in light of the results of studies of high-sensitivity troponin for risk prediction.
Developments in POC cardiac testing
Cardiac marker testing is performed in the hospital setting both in the central laboratory and at the point of care (POC). Demand for POC cardiac marker testing is growing as hospitals strive to meet the goals set forth in guidelines from the American Heart Association and the American College of Cardiology (Washington) that call for a maximum turnaround (vein-to-brain) time of 60 minutes, and a preferred turnaround time of 30 minutes. Many hospitals are unable to meet the more stringent 30 minute goal using cardiac marker testing performed in the central lab due to the delays involved in sample transport, sample log-in, analysis and reporting of results back to the floor. Point-of-care testing also eliminates issues with sample mix-up and with pre-analytical errors due to improper sample handling during transport.
A number of POC cardiac marker testing products are on the market in the U.S., as shown in Table 2. on the next page. Inverness Medical is the leader in the POC cardiac marker segment worldwide with the Triage product line acquired via its acquisition of Biosite. Roche Diagnostics is the No. 2 supplier, with a strong presence in the European market, followed by Siemens Healthcare Diagnostics with a 10% share of the market. Abbott Diagnostics has expanded its presence in the POC cardiac marker segment with the addition of Troponin I, CK-MB and BNP assays to its i-STAT analyzer menu. Sales of the i-STAT system are mainly concentrated in U.S. hospitals, but Abbott plans to expand outside the U.S., initially focusing on Germany and the UK in 2009.
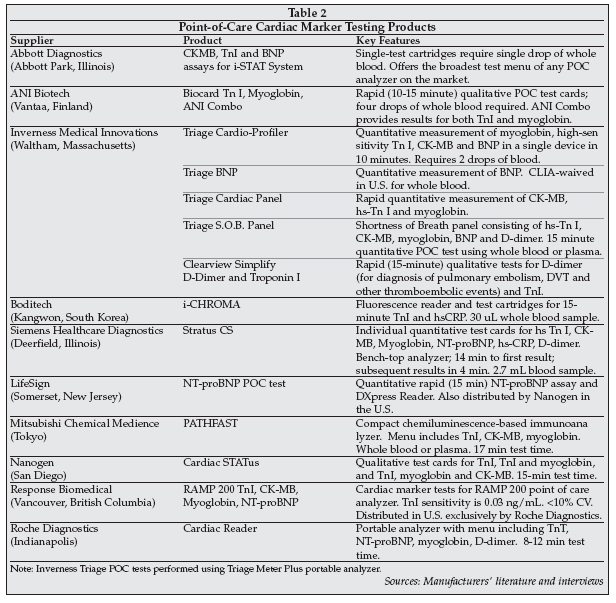
A number of new products for POC cardiac marker testing are under development. NanoEntek (Seoul, South Korea) exhibited the heArt2 analyzer at the AACC gathering, a development-stage system for rapid, point-of-care testing that is based on lateral flow immunoassay technology in a biochip format with fluorescence detection. The initial test menu will include Troponin I, CK-MB and myoglobin, with a test time of five minutes and a whole blood sample volume requirement of less than 20 uL. The company plans to obtain a CE mark for the product by September or October, and will launch the system in October outside the U.S.
NanoEntek is looking for partners to distribute the product in the U.S., and will seek FDA clearance. The company plans to add an NT-proBNP assay to the test menu by the end of 2008, and will also develop a test for D-dimer. The analyzer includes an LIS interface and barcode scanner, as well as an optional printer. Cost per test is expected to be lower than for POC cardiac marker tests now on the market.
Nanosphere (Northbrook, Illinois) has developed a research-use, high-sensitivity Troponin I assay for its Verigene system, a combination nucleic acid/protein analyzer designed for decentralized laboratories in the hospital. The Verigene system uses gold nanoparticle technology, microfluidics, and multiplex array analysis to perform high-sensitivity assays in single-use disposable test cartridges. Nucleic acid tests for F2 and F5 gene mutations associated with coagulation disorders, as well as a genetic test for MTHFR polymorphisms linked to hyperhomocysteinemia and elevated risk of thromboembolism, have already been introduced for in-vitro diagnostic use on the Verigene system. The high-sensitivity Troponin I assay will use the same test format as is used for the DNA tests, and will have sensitivity in the low picogram per ml range. Introduction of the Verigene troponin assay is targeted for 2009.
e2v Biosensors (Essex, UK) is developing a new POC testing platform based on surface-enhanced Raman spectroscopy biosensor technology. The company is a developer of sensor and detector products for a wide range of applications in the medical, bioscience, aerospace, defense, commercial and industrial markets. The first assay under development is a high-sensitivity C-reactive protein test, to be followed by Troponin I. A sensitivity of 5 pg/mL has been demonstrated for hs-CRP in a 10 minute assay, and similar performance is expected for the troponin assay.
New risk assessment approaches
In addition to their use in initial diagnosis of acute coronary syndromes and heart failure as well as prognosis following an event, cardiovascular disease markers are being employed increasingly for assessing disease risk in the population. Lipid panels including total cholesterol tests are widely used in health screening to detect atherosclerosis and coronary heart disease, and newer markers such as hs-CRP are being used increasingly to complement lipid testing for improved detection of cardiovascular disease.
Now, as discussed in multiple presentations at the AACC conference, molecular diagnostics is beginning to be investigated as a new tool to improve detection of cardiovascular disease. Since less than half of individuals at risk for a coronary event are identified by traditional risk factors, improved detection methods clearly are needed. At an AACC plenary session, Elizabeth Blackburn, PhD, of the University of California, San Francisco, discussed the potential for using telomerase testing as a new approach for cardiovascular disease risk assessment.
Telomerase is an enzyme responsible for protection of telomeres, which are in turn involved in protecting chromosomes from loss of genetic information. Telomerase also is found in high levels in cancer cells, and when initially discovered the enzyme was thought to be a potential cancer marker. However, the development of more sensitive assays for telomerase has enabled detection of the levels of expression in normal tissue, and it is now recognized that low levels of telomerase are correlated with heightened disease susceptibility. Shortened telomeres are associated with increased mortality rates for all diseases, including a 3.2-fold increase in cardiovascular mortality.
Blackburn has found that low telomerase levels are associated with six known risk factors for cardiovascular disease, including chronic stress. At present, however, assay methods for telomerase need to be improved in order to determine if the enzyme is a useful risk marker. Existing assays measure telomerase levels in peripheral blood mononuclear cells, requiring a separation step and well-controlled blood drawing methodology. There is the possibility that isolation of a certain mononuclear cell type for telomerase determinations could improve diagnostic accuracy, serving as a signature cell type for risk assessment.
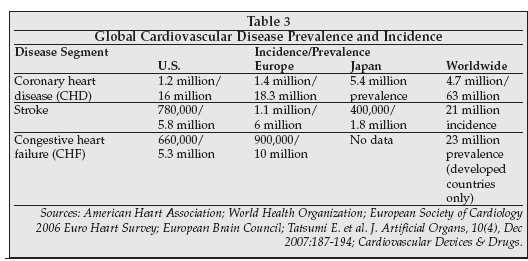
Genetic factors involved in cardiovascular disease risk and response to therapy were also discussed at AACC. Cardiovascular disease is a global health problem, as indicated by the data in Table 3, but incidence and prevalence rates vary substantially between different populations. Environmental factors such as diet, lifestyle, and smoking play a substantial role in disease causation, but genetic factors also appear to be important.
Genetic factors explain only a small part of the variation between populations, however. For example, as discussed by Lynne Jorde, PhD, of the University of Utah (Salt Lake City) at an AACC plenary session on the biomedical implications of genetics and race, 90% of all genetic variations are observed within a given population (e.g., European, African, Asian) while only 10% are observed between populations. As a result, analysis of genetic variations is probably more important in determining factors such as disease risk and response to therapy than race or ancestry.
Citing an example relevant to cardiovascular disease, Jorde said that some individuals have genetic patterns or variants of the angiotensin gene, a gene involved in blood pressure regulation, that are more similar to those of a different population or race than the one to which the individual belongs. As a result, molecular genetic testing is likely to prove to be a key enabler of personalized medicine in cardiovascular disease, as well as in the management of a number of other chronic diseases such as cancer, diabetes, and neurological disease.
A number of companies exhibited products for molecular diagnostic testing with cardiovascular disease applications at the conference. Osmetech Molecular Diagnostics (Pasadena, California) has received FDA clearance for a warfarin sensitivity test for its eSensor XT-8 analyzer, a random access microfluidic cartridge-based system that provides four-hour turnaround time using off-line PCR and electronic microsensor detection. The test detects the *2 and *3 variants of CYP2C9 and the -1639A VKORC1 variant, presence of which indicates that a lower warfarin dose is indicated. An expanded warfarin response panel is in development, as are tests for respiratory viruses and cystic fibrosis screening.
AutoGenomics (Carlsbad, California) also exhibited molecular genetic tests for its Infiniti analyzer that have applications in cardiovascular disease. Tests for Factor II and Factor V as well as a Factor II-Factor V panel are available for IVD use in evaluation of patients with suspected thrombophilia, as is a pharmacogenomic test for warfarin sensitivity.
