CDU Contributing Editor
ATLANTA, Georgia – New technologies often drive major changes in the various segments of the medical device market, resulting in the emergence of new competitors and altering the way in which patients are managed. The cardiology sector is perhaps the best example of a technology-driven medical device market, with major new innovations occurring at a rapid pace. One area of that market that has attracted considerable attention recently is new approaches to the management of patients with congestive heart failure (CHF). A number of companies are beginning to use advanced remote monitoring technologies and information systems for CHF management. Related advances have emerged for management of patients with hypertension and cardiac arrhythmia. A key goal of many of the new approaches to disease monitoring is to prevent serious adverse events by detecting deterioration in patient condition earlier, allowing corrective action to be taken. Longer term, new approaches using personalized medicine may allow the course of disease to be predicted, and allow targeted therapies to be used to achieve improved outcomes.
The increased use of minimally invasive therapy is another important trend in cardiovascular disease management, providing the opportunity to implement treatments when disease or a change in disease course is detected at an earlier stage since the risk of intervention is lowered. Advances are continuing to emerge in minimally invasive bypass surgery, including the development of prosthetic grafts, in minimally invasive treatments for heart defects and in technologies that can help minimize the damage due to acute events such as myocardial infarction and, in the long term, other disorders including stroke. Many of the companies that are developing new technologies for cardiology demonstrated their most recent advances here at this year's annual scientific sessions of the American College of Cardiology (ACC; Bethesda, Maryland). While much of the emphasis at present in cardiology is on new developments in stents, a number of other areas offer attractive opportunities for suppliers.
Remote monitoring aids patient management
One new development in remote monitoring exhibited at the ACC sessions is a new consumer-oriented application of the CardioSentry from eHealthLink (Portland, Oregon). The CardioSentry is a compact post-event recorder that is used to store ECG data when a patient believes a heart arrhythmia is occurring. The data can be recorded by pushing a single button and subsequently is transmitted to a monitoring center for immediate analysis 24 hours a day, seven days a week. Trained cardiac technicians are available to consult with the patient to help interpret the transmitted ECG data. While such services have been available to physicians for patient management for a number of years, eHealthLink has introduced a direct-to-consumer service that is available over the counter, without a prescription. The patient pays $120 initially, of which $100 is refunded when the monitor is returned. A monthly fee of $49 covers the monitoring service. The consumer service is designed to provide monitoring for patients once the limited service covered by most insurers is complete. The company believes the low cost of the recorder and the relatively inexpensive service fee will entice many patients to continue monitoring for a longer interval, particularly for those patients who are concerned about recurrent events. The FDA has approved the eHealthLink direct-to-consumer service, but each user must have a physician of record before subscribing, so the cardiologist remains involved in the patient's care.
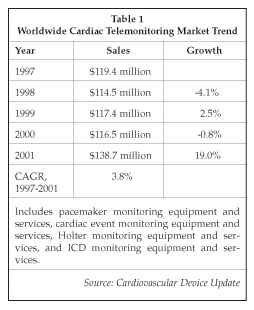
As shown in Table 1, the market for cardiac monitoring equipment and services is continuing to grow, although there has been a considerable degree of consolidation over the past few years, and some leading players have experienced a decline in revenues. Leading providers of cardiac event monitoring products and services include CardGuard AG (Schaffhausen, Switzerland) and Raytel Cardiac Services (Windsor, Connecticut). Raytel supplies products and services to 12,000 physicians throughout the U.S. using its telephonic and Internet-based ECG and Holter monitoring systems for patient management. The company also offers a transtelephonic ICD monitoring service that currently serves about 200 patients who have ICDs manufactured by St. Jude Medical (St. Paul, Minnesota). A new transtelephonic looping and event monitor, the CardioCall RT20, was introduced in 2001. The Raytel event monitoring service costs $640 a month. Raytel serves over 175,000 patients a year. However, sales in the cardiac monitoring segment have declined at more than 5% per year on average over the past four years. Mednet Healthcare Technologies (Ewing, New Jersey) is another supplier of transtelephonic cardiac event recording services that also offers pacemaker, Holter and resting ECG monitoring via telephone connections.
 |
CardGuard is the fastest-growing company in the cardiac monitoring segment, having completed numerous acquisitions over the past two years, including LifeWatch (Buffalo Grove, Illinois), Instromedix (San Diego, California) and Quality Diagnostic Systems. Sales increased from $11.8 million in 1999 to $93.3 million in 2001. CardGuard is expanding its monitoring services in Europe and other countries outside the U.S., and also plans to expand into other disease areas including diabetes, hypertension, new respiratory monitoring applications and fetal/maternal monitoring. Aerotel Medical Systems (Holon, Israel) is another provider of remote ECG event monitors. The company's Heartline trans-telephonic ECG technology allows patients to obtain an immediate analysis of their condition and also allows the physician to consult with a cardiologist about their patient's status. Aerotel also offers trans-telephonic devices for remote monitoring of blood pressure, respiration, glucose levels and body weight (for management of CHF patients). Another supplier of remote blood pressure monitoring devices, Wellness Monitoring (San Ramon, California), has introduced the BPfone in the U.S. The device, manufactured in Germany by Medical Monitors, is used by thousands of patients in the UK and Australia, and the company is now targeting the U.S. market as the next area for expansion. A monitoring center has been established in San Ramon that will serve patients throughout the U.S. via toll-free number. Remote, patient-directed monitoring of blood pressure is preferred over ambulatory blood pressure monitoring by patients, according to the company, because the patient maintains control over the monitoring process. Periodic reports on blood pressure trends are generated by the monitoring center and sent to the physician, who then reviews them during the patient visit. Eventually, Wellness Monitoring will add monitoring of glucose, body weight and other parameters that are available now outside the U.S., addressing additional patient populations including diabetics and CHF patients.
Advances in information systems technology also are having an impact on cardiology, allowing efficient capture of patient data from new locations such as the cath lab and outpatient clinics. For example, NextGen Healthcare Information Systems (Horsham, Pennsylvania), a unit of Quality Systems (Tustin, California), has introduced an electronic medical record system that can be accessed via a personal data assistant (PDA) with wireless Internet access to allow data to be recorded and accessed by a cardiologist in the cath lab, and that integrates all functions including charge capture, prescription ordering, order entry and patient data capture in a single device. The system also can be accessed from desktop computers. Cost is approximately $10,000 per physician user, plus an annual fee of 15% of the initial system price. The system also can be implemented via an application service provider (ASP) model at lower cost. Another system being marketed for cardiology applications that allows access via a palm-type computer is the reViewMD from Medcon Telemedicine Technology (Whippany, New Jersey). The system allows images from a variety of sources, including coronary angiograms, echocardiograms, MRI and CT scans, X-rays and images of electronic medical record documents to be viewed remotely via a PDA. The company has installed approximately 300 systems worldwide, including 50 in the U.S. in about one year. More than 180 cardiac image management systems are installed worldwide. Medcon also provides systems offering telecardiology and web-based features.
Technological advances also are changing the way in which vital signs monitoring is performed in cardiology and other disciplines. Philips Medical Systems (Eindhoven, the Netherlands) now offers a number of compact, portable vital signs monitors for hospital and alternate-site use, including the Telemon, a telemetry module that plugs into a bedside unit to display 5-lead ECG (and a simulated 12-lead ECG), along with pulse oximetry and non-invasive blood pressure. Other compact monitors from Philips include the A1 and A3, providing 3-lead and 2-lead ECG along with oximetry, noninvasive blood pressure and pulse rate in a portable package. There also is growing emphasis on technologies that can help to monitor brain ischemia in patients undergoing coronary artery bypass surgery as a result of recent studies indicating that procedure-related neurological damage may be more severe than believed. Somanetics (Troy, Michigan) is now finding some receptivity for its Invos brain oxygen saturation monitor in cardiac surgery because of data that shows a variety of deleterious effects of bypass surgery that may be preventable if adequate oxygenation is maintained. The system may also have applications in patients undergoing defibrillation. Philips recently introduced a new Portal attachment for its Merlin monitors, of which 170,000 are installed worldwide, that will allow testing to be performed at the bedside using the IRMA blood gas/electrolyte/chemistry measurement cartridges from Diametrics Medical (St. Paul, Minnesota). Cardiac surgeons continue to lose patients to interventional cardiology as advances in coronary stenting make transcatheter therapy an attractive alternative, particularly in light of growing evidence of brain damage as a result of stopped heart bypass surgery. As a result, there is growing demand for diagnostic systems that can monitor oxygenation status in the brain to help minimize ischemic damage.
Improved techniques shorten hospital stay
New developments in less-invasive therapy for cardiovascular disease continue to drive changes in the competitive structure of the market, as well as stimulating growth in utilization at the expense of traditional open surgical methods. For example, St. Jude Medical has achieved significant penetration with its Symmetry Bypass System Aortic Connector in the U.S. market following FDA approval of the device in May of last year. The Symmetry is an automated anastomosis device that minimizes manipulation of the aorta during coronary artery bypass graft procedures and reduces the risk of embolization that can lead to ischemia in the brain and other organs. Since the device was introduced, more than 23,000 implants have occurred worldwide. While the Symmetry can be used in open-chest surgery, it offers significant advantages when performing minimally invasive bypass surgery. Other companies marketing or developing automated anastomosis devices include Jomed (Helsingborg, Sweden); Cardiovations, a unit of Ethicon/J&J (Cincinnati, Ohio); Ventrica (Fremont, California), Guidant (Indianapolis, Indiana); HeartStent (Minneapolis, Minnesota); Auto Suture (Norwalk, Connecticut); Coalescent Technology (Sunnyvale, California); and Abbott/ Perclose (Abbott Park, Illinois).
Another focus for reducing the invasiveness of coronary artery bypass graft procedures is the development of prosthetic grafts, which could allow surgeons to eliminate the harvesting of saphenous vein from the leg or arterial conduits such as the internal mammary artery. The harvesting procedure represents the most invasive aspect of coronary artery bypass surgery for some patients, particularly when closed chest, minimally invasive technique are employed. The leader in the race to develop a synthetic vascular graft for coronary bypass, Thoratec (Pleasanton, California), announced in December 2001 that the FDA had granted it permission to begin a Phase II trial of the ARIA prosthetic coronary graft, following promising results in Phase I studies involving 19 patients at six centers. The trial is being expanded to include 20 centers and up to 162 patients. Thoratec is now targeting completion of the ARIA development program and FDA approval of the device sometime in 2004, with additional development costs estimated at $4.7 million. The company estimates the market opportunity for the device at in excess of $1 billion, based on a target patient population of about 20% of the patients who undergo coronary artery bypass surgery, or about 180,000 patients worldwide.
Another emerging market opportunity in minimally invasive cardiac therapy that may represent a substantial new segment of the transcatheter device market is devices for closure of atrial-septal defects. Another septal defect that occurs in adults is the patent foramen ovale (PFO), a transient hole that may open in the heart under physical strain, and that is associated with an increased risk for embolic stroke. Studies described at the ACC sessions showed that closure of such defects with a sealing device delivered via percutaneous transcatheter techniques results in a statistically significant improvement in outcome, including a reduction in the rate of major strokes as compared to medical treatment. Differences in PFOs and atrial-septal defects have led to the development of dedicated devices for PFO closure, including the PFO Star and the Amplatzer PFO Occluder. One study, described at the ACC sessions by Markus Schwerzmann of Swiss Cardiovascular Center (Bern, Switzerland), found fewer adverse events with the Amplatzer than with the Star occluder. Another study by the same group involving 150 patients treated with a closure device and 161 treated medically (with oral anticoagulation) found that none of the device-treated patients suffered a major stroke at 2.2 years mean follow-up, vs. seven patients who had a stroke in the medical therapy group. Many physicians have recently begun to advocate surgical closure of such defects because of the high risk of stroke. However, the results of the Bern studies show that good outcomes can be achieved using a percutaneous device, with considerably lower procedural risk.
As shown in Table 2, estimates of the size of the potential worldwide market for atrial-septal defect and PFO closure devices range as high as $1 billion. Some suppliers believe the market could be even larger, since many PFOs are left untreated because the risks of the open-heart surgical procedure are too great. One of the leaders in the segment at present is Nitinol Medical Technologies (Boston, Massachusetts), which acquired exclusive rights to the CardioSeal Septal Occluder in 1996. In 1998, the company introduced the STARflex Centering System to aid in implantation of the CardioSeal. The CardioSeal device is comprised of an MP35 wire frame (employing the same material as is used in pacemaker leads) with a Dacron covering. The current version of the CardioSeal uses a different wire type in order to resolve problems with fractures that occurred in the original design. Other companies developing devices for PFO and atrial-septal defect closure include Microvena (White Bear Lake, Minnesota), AGA Medical (Golden Valley, Minnesota), Dr. Osypka GmbH (Rheinfelden-Herten, Germany), W.L. Gore & Associates (Flagstaff, Arizona) and Pediatric Cardiology Medical Devices. AGA Medical is the manufacturer of the Amplatzer device family, which includes the Amplatzer Septal Occluder that is now FDA-approved for transcatheter closure of the atrial-septal defects; the Amplatzer PFO Occluder, approved under a humanitarian device exemption for PFO closure; and two development-stage devices for closure of patent ductus arteriosis and muscular ventricular septal defects. W.L. Gore, the global leader in the ePTFE vascular graft market, has introduced the Helex Septal Occluder for transcatheter closure of atrial-septal defects and PFOs in Europe. The Helex is under investigational status in the U.S. The Gore device consists of a nitinol wire frame covered by ePTFE, and is priced at EUR 5,000. Once implanted, it bridges the defect and eventually occludes it as coagulated blood and eventually tissue overgrowth close the pores in the ePTFE material. Gore estimates the potential worldwide market for its device at 80,000 to 100,000 units per year.
 |
New minimally invasive approaches for the treatment of coronary artery occlusive disease also continue to be developed, in spite of the major breakthroughs that have occurred in drug-eluting coronary stent technology. CryoVascular Systems (Los Gatos, California) described the results of animal studies using its CryoPlasty System, which consists of an angioplasty balloon chilled by nitrous oxide. Previous studies indicated that freezing of arterial tissues in vivo results in a benign healing process without significant neointimal tissue proliferation, indicating that restenosis might be minimized. The study using the CryoPlasty system, conducted at Stanford University (Palo Alto, California), showed no loss of lumen diameter and no increase in the degree of stenosis at any time interval following the angioplasty procedure. The entire cooling procedure requires about one minute, and multiple applications can be performed in a single patient. The technology results in a stent-like vessel configuration following angioplasty, in spite of the lack of placement of a stent, which is maintained over time. The company has received a CE mark for the product in Europe, and has received an IDE to begin Phase 1 trials in the U.S. The first patients were enrolled in the trial in July 2001.
Another catheter-based technology that continues to show promise in the treatment of acute coronary syndromes is under development by TherOx (Irvine, California). The TherOx AO system hyperoxygenates a patient's blood with aqueous oxygen and delivers the blood to ischemic regions following a myocardial infarction. The device is intended for use following primary angioplasty to salvage the maximum amount of myocardial tissue and provide improved left ventricular function compared to angioplasty alone. A Phase II study was initiated early this year, and results have so far been positive. The study will involve 250 to 300 patients at multiple centers. The TherOx AO system also received a CE mark in Europe in October 2001 after positive results were obtained in studies there. The device appears to be an important adjunct in the treatment of patients who have had a heart attack and presented to a treatment center within 24 hours of onset of symptoms. TherOx also is investigating applications of the system in stroke treatment, cancer therapy, wound treatment and skin care.
