BBI Contributing Writer
SAN DIEGO, California — The 31st annual congress of the Society of Critical Care Medicine (SCCM; Des Plaines, Illinois), held here in late January, provided a forum for the introduction of a number of new products for use in the critical care setting, as well as a window on the future of patient management in intensive care.
A key theme for the meeting, which attracted over 4,500 attendees, was the impact of new developments in genomics in critical care medicine. Although the relevance of genomics for patient management in the intensive care unit (ICU) may not be as obvious as, for example, in the diagnosis and treatment of genetic disease, recent studies indicate that a patient's genetic predisposition may play a very important role in determining outcome, including the likelihood of surviving a stay in the ICU.
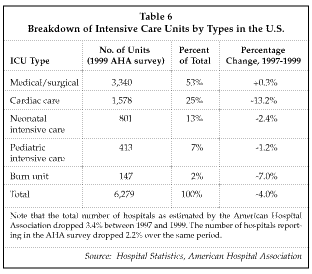
Another important topic addressed at the congress, and strongly emphasized by the society, is workforce issues in the ICU, including staffing shortages as well as the way in which critical care is structured in the U.S. The need for an improved organizational structure and for better approaches to information management in the ICU is expected to create opportunities not only for intensivists who deliver care to critically ill patients, but also for suppliers of critical care information systems. Although, as shown in Table 6, the number of ICUs is not growing overall, and is declining in some categories, occupancy rates are high and increasing in some cases.
 |
One of the most challenging tasks for intensivists practicing critical care medicine is the management of patients with sepsis. Sepsis is a large and growing problem in medicine. There are about 800,000 cases of sepsis annually in the U.S., with half of those classified as severe, and the condition is the second-leading cause of death in ICUs in the U.S. There is a significant need for improved markers for sepsis, to help detect the condition early before it becomes irreversible, as well as for improved therapies. Recently, a new drug for the treatment of sepsis, Xigris, was introduced by Eli Lilly and Co. (Indianapolis, Indiana), culminating many years of effort by numerous companies, and considerable investment, that had until now failed to produce an effective agent. The progress in sepsis treatment is expected to increase the demand for new technologies for early detection, as well as for technologies for monitoring treatment.
Advances were announced at the SCCM gathering in monitoring technologies for other types of disorders, including techniques for monitoring patients with cardiovascular disease, neurological disease, and trauma.
Another important area of development in critical care medicine is technology for organ and tissue preservation and support. The use of hypothermia to reduce the complications associated with fever is one key focus, as is re-warming of patients in surgery. At least three companies have developed systems for modifying patient temperature for use in cooling patients with fever in the ICU, or for re-warming surgical patients in the operating room. Advances also are continuing in technologies for temporary support of patients with life-threatening kidney and liver disorders.
Genomics, proteomics to revolutionize ICU
Genomics and the related field of proteomics potentially have a great deal to offer intensivists in the ICU for managing critically ill patients. As described by Tim Buchman, MD, PhD, professor of surgery, anesthesiology and medicine at Washington University Medical Center (St. Louis, Missouri), critical illness typically involves changes in expression of multiple genes, resulting in the development of conditions such as septic shock, acute respiratory distress syndrome and multiple organ dysfunction syndrome. Those conditions represent the patient's response to organ injury, infection or organ failure due to diseases such as cancer, and can be quite complex and difficult to diagnose. The definition of sepsis, for example, continues to be the topic of debate within the critical care medicine community. If not treated properly, the conditions often lead to rapid deterioration of the patient and death. Genomic testing, using technologies such as microarrays that allow simultaneous analysis of the expression of multiple genes, offers a powerful new tool that is already proving useful in identifying patients who are at highest risk of an adverse outcome in the ICU. According to Buchman, studies have shown that the rate of death due to infection in children can be 5.8 times higher as a result of genetic predisposition. Studies of gene patterns (Variable Number of Tandem Repeat or VNTR genotype) in ICU patients have shown a 94% mortality rate for those sharing one pattern vs. 32% for those with a different, more favorable, pattern.
One indicator of the increasing interest in using genomics to guide therapy in the ICU is a conference that is being organized by the National Institutes of Health (NIH; Bethesda, Maryland) in April addressing the topic of functional genomics of critical illness and injury. According to Buchman, the NIH also is sponsoring a clinical trial to assess genetic effects in a group of 8,000 critical care patients.
At present, technologies for performing genomic analysis needed in the critical care setting are only available for investigational use, although at some institutions there is already a considerable level of testing performed, with turnaround times of 24 hours in some cases. The GeneChip technology developed by Affymetrix (Santa Clara, California) is used by some investigators to profile critical care patients. Other companies marketing or developing genomic profiling technologies for research use include Nanogen (San Diego, California), Third Wave Technologies (Madison, Wisconsin), Aclara Biosciences (Mountain View, California), Molecular Staging (New Haven, Connecticut), Xanthon (Research Triangle Park, North Carolina), Genometrix (The Woodlands, Texas) and Roche Diagnostics (Basel, Switzerland), the latter in partnership with CombiMatrix (Mukilteo, Washington).
Major suppliers of nucleic acid diagnostic systems for the clinical lab have not announced the development of tests with specific applications in critical care genomics, with the current focus in the market remaining in the traditional infectious disease segment. Before genomic testing in critical care can enter the clinical diagnostics arena, it will be necessary to validate genetic markers that can be used to guide therapy, a development that will require large-scale clinical studies. The market opportunity is substantial, however. About 5 million patients are treated in ICUs in the U.S. each year, and the cost of critical care in an ICU comprises about 28% of total hospital costs for acute care.
Sepsis diagnosis and treatment
Considerable attention is now focused on the diagnosis and treatment of sepsis and septic shock in critical care medicine. The introduction of Lilly's Xigris provides intensivists with a new tool for the treatment of sepsis and has heightened interest in methods both to diagnose the condition earlier and to follow patient status during therapy. In the PROWESS trial to evaluate Xigris, mortality in a high-risk group of sepsis patients was reduced to 24.7% vs. 30.8% in controls. The drug, a human recombinant form of activated protein C, was approved Nov. 21, 2001, and had already generated sales of $21.2 million by Dec. 31. As discussed by L. Moldawer, MD, of the University of Florida College of Medicine (Gainesville, Florida), at the SCMM gathering, the use of gene therapy using viral vectors is also being evaluated for sepsis treatment to help to further reduce mortality. In particular, gene therapy to stimulate production of Interleukin-10 is being studied as a means to reduce the over-reaction of the immune system that is a hallmark of sepsis. Another tactic has been to use agents to inhibit coagulation in sepsis patients, since disseminated intravascular coagulation (DIC), in some cases with associated multiple organ failure, is another key characteristic of the syndrome. However, so far approaches to inhibit coagulation such as infusion of anti-thrombin III have not proven effective.
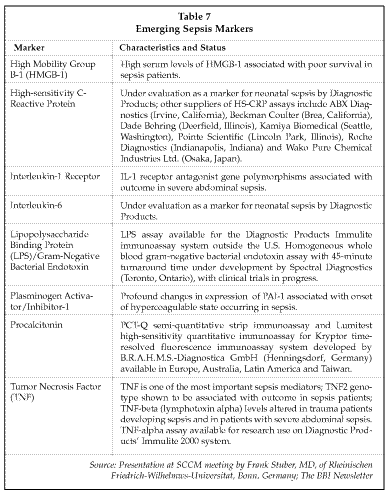
A number of new tools for sepsis diagnosis are under development, including genomic analysis as well as immunoassays for sepsis markers. As discussed at SCCM by Frank Stuber, MD, PhD, of Rheinischen Friedrich-Wilhelms-Universitat (Bonn, Germany) at the SCCM meeting, some of the key markers under study include catecholamine receptors, cytokine receptors (particularly interleukin receptors), glycoprotein levels on the surface of platelets, and Tumor Necrosis Factor. A list of existing and emerging sepsis markers is presented in Table 7.
 |
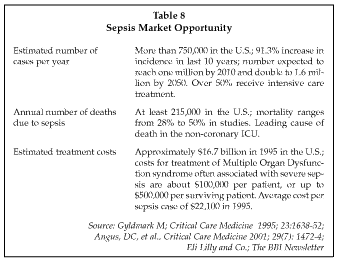
Diagnostic Products (Los Angeles, California) is one of the key suppliers of commercial immunoassays with applications in sepsis diagnosis and management, with a number of markers available on its Immulite immunoassay system. The company has shipped more than 6,700 Immulite systems worldwide, and Immulite sales now comprise 81% of the company's total sales, which increased 12% to $67.9 million in the third quarter of 2001. The market opportunity for sepsis tests and therapies is substantial, as shown in Table 8. Since the incidence of sepsis is highest in older patients, the aging of the population in the U.S. and other developed countries is expected to drive further increases in demand in the future, with the number of cases in the U.S. expected to double by 2050. Other factors driving the increase in sepsis incidence include the growing number of antibiotic resistant microorganisms and the use of more complex surgical procedures and more aggressive anti-cancer therapies.
 |
Some leading experts involved in the treatment of sepsis patients, such as Buchman, believe that the use of genomic testing at the bedside to assess a patient's susceptibility to sepsis and their risk of death if sepsis develops will become routine within the next five to seven years. Genomic tests already are being performed in the central laboratory at Buchman's institution with turnaround times as short as 24 hours, using technologies such as the GeneChip from Affymetrix. However, in order to implement such testing in the ICU setting, it will be necessary to solve quality control issues and simplify the analytical technologies. Such testing will allow physicians to individualize treatment based on a patient's predicted response, and may even allow patients to be managed differently, depending on their risk of an adverse outcome from the time they enter the ICU. Key companies marketing or developing assays useful in sepsis management include B.R.A.H.M.S. GmbH, Diagnostic Products, and Spectral Diagnostics (Toronto, Ontario). B.R.A.H.M.S. and Spectral both are offering or developing point-of-care testing platforms (qualitative rapid tests) that can be used for bedside determinations of sepsis markers. Diagnostic Products offers laboratory-based test systems but provides a wider range of tests for sepsis management.
Advances in critical care monitoring
Another area of continuing development in critical care medicine is patient monitoring technologies, including technology for cardiac and hemodynamic monitoring and neurological monitoring technologies. Edwards Lifesciences (Irvine, California) has developed volumetric technology for continuous cardiac output monitoring that allows direct measurement of the right ventricular end diastolic volume index (RVEDVI). As discussed by R.P. Dellinger, MD, of Rush Presbyterian-St. Luke's Medical Center (Chicago, Illinois) at the SCCM meeting, the index in conjunction with right ventricular ejection fraction is proving superior to traditional parameters such as pulmonary artery wedge pressure and central venous pressure in predicting a patient's response to resuscitation therapy in the ICU. Such monitoring is proving useful in the management of patients with sepsis and acute respiratory distress syndrome (ARDS). Studies using the index also have elucidated some of the issues surrounding use of PAW and CVP in the ICU, including concerns raised in prior studies about excess mortality in patients receiving pulmonary artery catheters. Michael Cheatham, MD, of Orlando, Florida, said the problems encountered in the past with pulmonary artery catheter use probably were the result of relying only on pressure measurements to interpret hemodynamic function, rather than including volume information as well. The consensus is that using only PAW or CVP is no longer the standard of care in the ICU, and that inclusion of a volume index is essential in order to properly evaluate hemodynamics.
Interest also is growing in monitoring of metabolic parameters in critical care. Lactate is being employed by an increasing number of critical care physicians to monitor metabolism and as an early indicator of conditions such acidosis or bacterial meningitis. Nova Biomedical (Waltham, Massachusetts) now offers a lactate assay on its compact pHOx analyzer for use in point-of-care testing. The pHOx is the company's best-selling product. Datex-Ohmeda (Madison, Wisconsin) has introduced a new multi-disciplinary monitor, the S15 Critical Care Monitor, that provides pulmonary, cardiovascular, electroencephalography, gastric perfusion and nutrition monitoring in a single integrated system. The new monitor both provides a simplified approach to metabolic monitoring and helps physicians assess the impact of patient nutrition on outcome. New technologies to monitor tissue metabolism and microvascular blood flow, including measurement of tissue pH and pO2 as well as oxygen saturation patterns, are being evaluated by researchers for use in assessing patients in surgery as well as in patients in the ICU with sepsis, ARDS and other critical conditions. As described by D. DeBacker of Erasme University Hospital (Brussels, Belgium) at the SCCM conference, measurements of microvascular flow patterns have shown that changes are correlated with outcome in sepsis. Cytometrics (Philadelphia, Pennsylvania) has developed the Hemoscan system for noninvasive measurement of tissue perfusion patterns, with applications in monitoring of shock in intensive care. The Cytometrics system uses a technique called orthogonal polarization spectral (OPS) imaging, or microvideoscopy, to measurement heterogeneity of tissue oxygenation. Measurements in patients with cardiogenic and septic shock demonstrate considerable heterogeneity in tissue flow, with some vessels having high flow while others have almost none.
Another new development in monitoring was unveiled by Metracor Technologies (San Diego, California). The company has developed the RODA Monitor, which combines the blood gas/electrolyte monitoring technology developed by the former VIA Medical with hemodynamic monitoring technology developed by TNO Biomedical (Amsterdam, the Netherlands), with additional involvement and funding from Alliance Pharmaceutical (San Diego, California). TNO Biomedical's continuous cardiac output (CCO) measurement technology employs arterial pressure pulse wave analysis, a technique that can be used in place of thermodilution after a calibration has been performed. The RODA adds ionized calcium to the VIA sensor cartridge in addition to the integration of CCO capability and other derived hemodynamic parameters. New hardware/software allows the sensor cartridge and arterial line to travel with the patient and plug into monitors at multiple locations, along with a sophisticated graphical user interface that allows the clinician or nurse to rapidly interpret patient status. The system recently received FDA 510(k) approval, and availability is projected for this summer. The added features of the RODA system should help Metracor expand beyond the 60 institutions now using the monitor developed by VIA Medical. The existing sensor for measurement of blood gases and chemistry parameters is priced at $200 ($300 for the neonatal version), and can be used for three days. The new RODA sensor will be priced at $250. In order to calibrate the RODA hemodynamic monitor, the user inputs patient height, weight and age, and parameters are computed that relate the arterial pressure wave measurements to thermodilution cardiac output. For blood gas and chemistry measurements, including measurement of pO2 , pCO2 , pH, sodium, potassium and hematocrit, the system intermittently pulls between 1.5 ml and 2.5 ml of blood from the radial artery into its sensor compartment, performs a measurement and then returns the blood to the patient along with an additional one cc of fluid. About one minute is required to perform the analysis, and a measurement can be performed every ten minutes. Monitors are typically placed free of charge in return for use of a certain minimum number of sensors.
Hemodynamic monitoring
Cardiodynamics International (San Diego, California) continues to increase its penetration in the hemodynamic monitoring segment with its BioZ noninvasive cardiac output system. The BioZ employs impedance cardiography technology to measure cardiac output, systemic vascular resistance, contractility and fluid level. Reimbursement for Medicare patients is now offered for a number of indications, including monitoring of suspected cardiovascular disease, fluid management in cardiac patients, determination of the need for IV inotropic therapy and monitoring of heart-transplant patients after myocardial biopsy. The system is marketed through a partnership with GE Medical Systems (Waukesha, Wisconsin), and modules also have been developed for the GE Solar series monitors that connect to the BioZ leads. For 2001, Cardiodynamics reported sales of $19.6 million, an increase of 50% vs. 2000. The BioZ installed base exceeds 1,650 worldwide, up 50% from a year ago. The company reported a profit in each of the past two quarters.
A new cardiac output monitor was introduced by LidCO Ltd. (Cambridge, United Kingdom) at the SCCM congress. LidCO's PulseCO System employs arterial pressure wave analysis to derive a beat-to-beat cardiac output reading, which is calibrated using a unique new lithium dilution technique. The PulseCO analyzes the signal from a radial artery pressure sensor to derive cardiac output. At the start of monitoring, a subtherapeutic dose of lithium chloride is injected in the venous circulation, and an ion-selective electrode sensor in the arterial line is used to measure the dilution curve and calibrate the system. The lithium dose is 240-fold lower than the standard dose given for manic depression. According to LidCO, the lithium dilution method is three times more accurate than thermodilution, providing a highly precise calibration that is maintained for eight to 12 hours. The system has been on the market for less than one year, and 128 have been placed worldwide, including 65 in the U.S. Sales have reached about $1.6 million. The PulseCO is priced at $12,000, with a disposable price of $109.
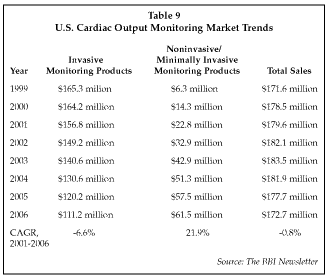
Additional systems for monitoring cardiac output were exhibited at SCCM by Pulsion AG (Munich, Germany) and Deltex Medical (Branford, Connecticut). The Pulsion PiCCO system uses a less-invasive monitoring technique using a femoral artery catheter and performs pulse contour analysis on thermodilution waveforms to generate a beat-to-beat cardiac output. Cost is about $150 per application. The Deltex CardioQ uses esophageal Doppler ultrasound measurements to derive a less-invasive cardiac output reading. The CardioQ monitor is priced at $8,000, and the probes are priced at $99 if the monitor is purchased, or $119 if the monitor is placed at no charge. The U.S. market for cardiac output monitoring products (Table 9) is substantial, with most of the growth in the noninvasive segment at present. The noninvasive segment is expected to continue to exhibit rapid growth for the next few years as additional new market segments develop. Cardiodynamics estimates that the total market opportunity, which includes applications in a variety of alternate site settings such as physician offices, clinics and, ultimately, home monitoring, represents an annual procedure volume of almost 30 times the existing market, indicating a large potential for market expansion.
 |
Neurological monitoring
Another important segment of the critical care monitoring products market is neurological monitoring systems. The existing field of neurological monitoring encompasses two primary segments, electroencephalography and intracranial pressure (ICP) monitoring. In addition, many patients with neurological conditions undergo MR imaging to characterize lesions in the brain, and certain biochemical markers such as neuron-specific enolase (NSE) and Protein S-100 may be employed. Another widely used technique, the xenon CT scan, was recently removed from the market because of inadequate FDA approval documentation. The market for ICP products is relatively flat at present, characterized by mature technologies. Studies have shown significantly lower mortality for patients managed with routine ICP monitoring and associated drainage of cerebrospinal fluid, but the trials have generally not been well controlled. Integra NeuroSciences (Plainsboro, New Jersey) is a major supplier of ICP monitoring systems, with the Ventrix Monitor employing fiber optic pressure sensing technology. Integra also markets the MoniTorr line of lumbar and ventricular catheters and monitoring systems. Another Integra product, the Licox, is used for monitoring of brain tissue oxygen levels in critical care patients. The Licox system originally was developed by GMSmbH, a German company acquired by Integra in April 2001 for $2.3 million. Worldwide sales of the Licox system were approximately $1.2 million in 2000. The Licox system was cleared for sale in the U.S. in January 2001. The system can be used to directly determine if the brain is receiving adequate levels of oxygen, rather than inferring oxygen status from measurements of blood pressure, intracranial pressure and peripheral blood oxygen saturation levels. More than 75 hospitals have either purchased the $12,000 system or are evaluating it. Another system for measurement of brain oxygen levels, and which also provides measurements of pCO2 and pH, is manufactured by Diametrics Medical (St. Paul, Minnesota) and marketed by the Codman (Raynham, Massachusets) division of Johnson & Johnson. However, monitoring of brain oxygen can prove difficult, since placement of the sensor probe is critical to obtaining meaningful readings, and there is no well-established technique to rely upon for determining the optimum location for the probe.
The newest addition to the array of neurological monitoring technologies is the bispectral index (BIS) monitor from Aspect Medical. The $9,500 BIS monitor is an electroencephalography system that analyzes electrical impulses via a $25 sensor placed on a patient's forehead. The BIS system computes a number between zero and 100 that represents the patient's level of consciousness and response to sedation. A value near 100 indicates the patient is awake, while a value of zero indicates the absence of brain electrical activity. In initial studies conducted in patients in the ICU, more than 69% of patients were found to be inappropriately sedated. Use of the BIS monitor has been shown to reduce spending on sedatives and to reduce patient recall of unpleasant experiences.
Aspect has licensed the BIS technology for integration into patient monitoring systems from most major suppliers, including Philips Medical Systems (Andover, Massachusetts), GE Medical Systems Information Technologies (Milwaukee, Wisconsin), Datex-Ohmeda, Nihon Kohden (Tokyo), Spacelabs Medical (Redmond, Washington) and Drager Medical (Telford, Pennsylvania). The latest version of the sensor employs four electrodes, with a new above-eye element designed to help reject artifacts. The system can monitor either the left or right side of the brain. Physiometrix (Billerica, Massachusetts) is another supplier of level of consciousness monitors and markets the PSA 4000 Patient State Analyzer, which can used to assess the level of anesthesia. The PSA 4000 also uses a four-lead EEG array and has been marketed in the U.S. for about a year. The system provides a global indication of brain activity vs. the hemispherical measurement provided by Aspect.
New innovations in the treatment of brain-injured patients are one factor helping to stimulate demand for neurological monitoring technologies. Monitoring of brain tissue oxygen levels is useful, for example, to identify patients in need of more aggressive oxygenation therapy to help prevent the additional tissue damage that can often result in head trauma patients due to pressure build-up and other mechanisms that restrict blood flow after the initial injury. Another common mechanism that can worsen tissue damage in patients with brain injury is high fever, which can increase the production of deleterious biochemical agents in brain tissue and contributes to the breakdown of the cytoskeleton of nerve cells in the brain.
Recently, new technologies for inducing hypothermia in brain-injured patients have been introduced to help control fever and improve outcomes. Alsius (Irvine, California) is developing the Coolgard 3000 for temperature management of patients in the ICU. A CE mark has been obtained for the Alsius system, and a 510(k) was filed in the U.S. recently. So far, the system has been used in about 550 patients worldwide. Applications include reducing the adverse effects of fever in trauma patients, as well as reducing tissue death in patients suffering from stroke or myocardial infarction. The system will be priced at about $25,000, and the disposable catheter used with the system costs $550. In total, Alsius estimates that about 800,000 patients worldwide could be candidates for use of the system. Other suppliers of systems for inducing hypothermia or for rewarming of patients after surgical procedures include Medivance (Louisville, Colorado), InnerCool Therapies (San Diego, California) and Radiant Medical (Redwood City, California).
