BioWorld Executive Editor
Editor's note: The following articles are compiled from the recently released BioWorld & Medical Device Daily Obesity Report: Tipping The Market Scales With Biotech & Med-Tech Regimens. For more information or to order a copy of the report, please visit www.bioworld.com/bioobesity.
The world is getting bigger in terms of weight, while the drug and medical technology markets that society has become reliant on to develop products for all the major illnesses that afflict it are trying to play catch-up and cope with a half-century of weight gain that threatens to clog society's major veins of productivity and commerce, along with its health.
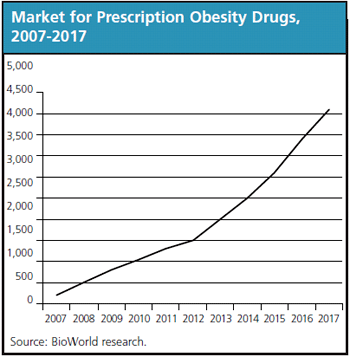
The weight management market, depending on which products, procedures or services are included, is valued in a range of $1.4 billion, which represents the current drug market, to $550 billion, which comprises the "kitchen sink" market, in which everything (from partial stomach excision to hypnotism to diet soda) that purports to address weight issues is included. Unfortunately, biopharma drugs account for a lackluster less-than-1 percent of the total market. Med-tech's contribution is better and increasing, but still in the same basement category of the overall obesity market.
Med-tech and pharma dominate the medically prescribed treatment market for obesity now, although pharma's stint at the top is more by default than a solid R&D aptitude on its part. Medical technology products and services are very effective in treating obesity, but its procedures often are regarded as "for extreme use only" and perceived to be inappropriate for many who likely would opt for an effective drug regimen over a more risky, albeit reliably effective, surgery.
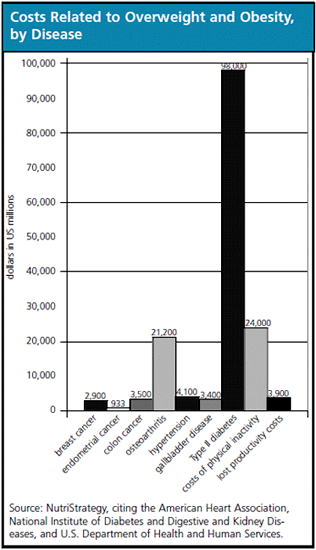
Approximately 80 percent of the hospitals surveyed for a study that was published in The Journal of the American Medical Association in January 2008 on the prevalence of obesity reported an increase in admissions of severely obese patients, reflecting a 22 percent increase from the previous year. Approximately half of those hospitals reported that they purchased new categories of supplies for the treatment of obese patients, including beds, lifts and wheelchairs.
At the moment, surgery is more effective in treating obesity than prescribed pharma drugs, and it has nothing to do with the difference in the number of drug products vs. the number of services and devices. While use of approved pharmaceuticals can reduce excess weight by 5 percent to 10 percent, patients undergoing obesity surgery lose 50 percent to 90 percent of their excess weight.
Pharma's less-than-blockbuster market run is helped by the absence of approved biotech products, but biotech looms to exploit the gaping hole in the treatment market, perhaps as early as late-2010. Whenever the leading crop of biotech applicants gains approval, they are immediately expected to find revenue success, manifesting the market's long-starved appetite and wholesale readiness for safer, more efficacious, easy-to-administer therapeutics.
Although med-tech research advances are hinting at the possibility of some innovative standalone and combination products that could enter the market within the next five years, the advantage of biotech products would seem to be the hope of manipulating cellular activity and "turning off" obesity triggers. Based on encouraging recent results from med-tech, such as the announcement in March 2010 that a team of California Institute of Technology researchers had successfully delivered cancer-fighting RNAi therapies to particular parts of the body via nanotech polymer robots, such advances in gene therapy could break down that technology's biggest barrier to success - patient site delivery of medication - and hasten the development of many stalled therapeutics in the clinic.
Such potential offers a respite from the inefficacy/side effect dilemma that beleaguers pharma drugs on the market as well as in the clinic, as well as the "drastic treatment" perception that frequently designates medical technology procedures and devices. "Drastic gastric" bariatric surgeries, temporary weight loss prescription drug results and OTC weight loss pills that all but come with an FDA recommendation to carry an extra set of pants, leave skepticism in patients and the door open for the biotechnology industry to ride in on a white horse with that magic pill solution that brings a higher and longer-term efficacy rate, reduces the need for surgeries and carries less ominous side effects than excessive flatulence and irrepressible diarrhea.
There are currently no approved biotech drugs in the space, but they loom, as do the revolutionary medical technologies that may overcome the delivery mechanism challenges that have thwarted advances in administering gene therapy biotech drugs to locations within the body.
There are currently 38 drugs in clinical trials for direct obesity applications, with 19 in late-stage trials and three filed for approval. That is not an impressive number, considering the hundreds of clinical trials being conducted for pervasive diseases such as cancer and heart disease; however, it is an indication that the drug development industry is not ignoring the unmet need. Twenty years ago, there was no serious or comparable clinical effort to address the growing indication. That had at least something to do with the perception that being overweight was regarded as a problem of personal overindulgence that could be addressed by changes in habit.
As illogical as it may seem, this problem (just like the weight often does on individuals) crept up on everyone, even though it unfolded right before our eyes. The opportunity to control the proliferation of obesity is still upon those industries, but its 50-year head start has given med-tech and biotech companies that deem to control its further spread an arduous, but far from impossible, task.