As Bluebird Bio Inc. soaked up most of the spotlight on gene therapy by opening the global phase III trial of its Lentiglobin BB305 candidate in patients with beta-thalassemia, stealthy Avexis Inc. sought to extend its cash runway by pricing an upsized public offering of 4.25 million common shares at $34.50 apiece. (See related story in this issue.)
The price represented a discount of approximately 2.5 percent to Wednesday's close of $35.41 for the company's shares (NASDAQ:AVXS). Chicago-based Avexis expects to realize $127.7 million in net proceeds by offering about 3.96 million of the shares – up from 3.57 million in its SEC filing – while investor PBM Capital Investments stands to collect $14.96 million from the sale of 433,526 shares.
Avexis granted underwriters a 30-day option to purchase up to 637,500 additional common shares, potentially generating another $22 million.
Goldman Sachs & Co. and Jefferies LLC are joint book-running managers for the offering, expected to close by Sept. 13, with BMO Capital Markets as lead manager and Chardan as co-manager.
Through a spokeswoman, Avexis declined comment, citing a quiet period. In its filing, the company said proceeds from the offering will be combined with existing cash and equivalents to fund the ongoing phase I trial of lead candidate, AVXS-101, in the initial indication of spinal muscular atrophy (SMA) type 1 and future studies in other forms of SMA and to finance manufacturing activities, including the establishment of its own manufacturing facility.
In its filing, Avexis reported $131.4 million in cash and an accumulated deficit of $95.1 million as of June 30.
Avexis, which began its corporate life in 2010 as Biolife Cell Bank LLC, converted in 2012 to a Delaware corporation, Biolife Cell Bank Inc., which housed two wholly owned subsidiaries that owned equipment and intellectual property related to its stem cell business. The company exited that space in 2014, rebranding as Avexis to pursue gene therapy.
Avexis set in motion a series of licensing deals that included an exclusive global agreement with Nationwide Children's Hospital (NCH), of Columbus, Ohio, for patent applications related to the intravenous and intrathecal delivery of AVXS-101 to treat all types of SMA. The company also inked an exclusive global license with Regenx Biosciences LLC (now Regenxbio Inc.), of Rockville, Md., for patents and applications to use its AAV9 capsid for in vivo gene therapy to treat SMA in humans. In addition, Avexis holds a nonexclusive global license agreement with Asklepios Biopharmaceutical Inc., of Chapel Hill, N.C., for patents and applications that apply to use its self-complementary DNA technology to treat SMA.
In February, the company priced 4.75 million common shares at $20 apiece to raise $95 million in its IPO. Avexis had the misfortune to begin trading on a day that saw a 2.4 percent drop in the Nasdaq Biotechnology Index, which dragged the company's shares down to a close of $18.05 for a loss of $1.95, or 9.8 percent. (See BioWorld Today, Feb. 12, 2016.)
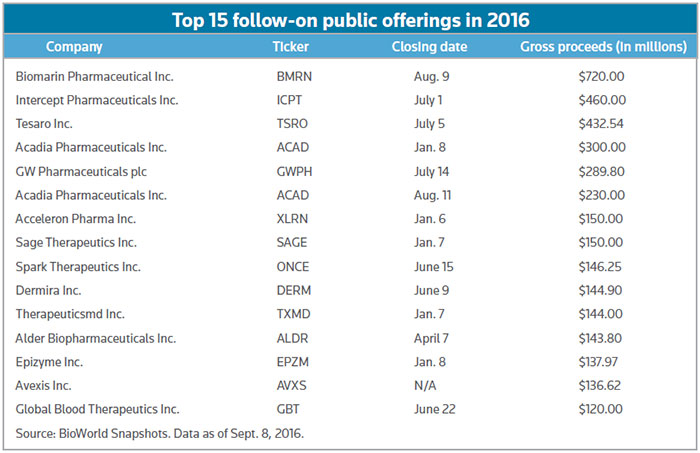
Despite that setback, shares have since doubled in value, and the follow-on ranks amid the top 15 of such public offerings this year – including separate rounds of $230 million and $300 million by Acadia Pharmaceuticals Inc. – according to the BioWorld Snapshots database. (See chart, below.)
IMPORTANT 'TO INTERVENE AS EARLY AS POSSIBLE' IN SMA
SMA is a neuromuscular disease caused by a genetic defect in the SMN1 gene that leads to the loss of motor neurons and results in progressive muscle weakness and paralysis. Avexis is targeting type 1, which causes 60 percent of SMA cases, because it represents the largest genetic cause of death in infants and has no approved treatments, despite an average life expectancy of less than two years.
A one-time intravenous treatment with AVX-101 is intended to deliver a fully functional human SMN gene into target motor neuron cells, where sufficient levels of SMN protein will be produced to improve motor neuron function, with rapid onset of effect in addition to sustained SMN protein expression.
Last month, the company reported that, as of July 1, AVXS-101 showed a favorable safety profile in the ongoing phase I trial, which fully enrolled 15 patients as of December 2015, with no new treatment-related safety or tolerability concerns. As of the cutoff date, patients in both the low- and proposed therapeutic dose cohorts remained without any events, defined as death or until a patient requires at least 16 hours per day of ventilation support for more than two weeks in the absence of acute reversible illness or following surgery. Mean motor function scores in both dosing cohorts continued to increase, with 25 percent of patients in the therapeutic dose cohort achieving motor function in a range considered to be normal.
In conjunction with the report of the company's second-quarter financials, Avexis disclosed that one patient in the low-dose cohort began to increase use of bi-level positive airway pressure in advance of surgery related to hypersalivation, a condition experienced by some SMA patients. Since the patient did not meet the definition of a permanent ventilation endpoint until after July 1, the independent data safety monitoring board (DSMB) said it would classify the development as an event in the subsequent data period. The event was determined by the DSMB to represent disease progression rather than a treatment-related adverse event (AE).
Avexis reported 107 AEs as of July 1, including 31 deemed serious AEs. Two of the SAEs were deemed treatment-related, consisting of clinically asymptomatic liver enzyme elevations.
Discussing the trial design, "the low dose was at the request of the FDA to help establish safety," Sean Nolan, the company's president and CEO, explained on the company's second-quarter call. "What we're seeing here is very much consistent with what we [saw] in the preclinical data, which [is] that there is a clear dose response with patients getting the therapeutic dose vs. those that are getting the lower dose."
The treatment of SMA type 1, he added, is likely to yield "degrees of success" that depend on the progression of the disease "and how many motor neurons are left to be rescued," in combination with the dosing regimen. "All this leads to how important it really is to intervene as early as possible in children suffering from SMA type 1," Nolan maintained.
Those factors have been taken into account in the phase III trial design, which has been in the works "for months and months," he admitted, including the potential prospect of an approval in SMA for nusinersen, the candidate from Biogen Inc. and Ionis Pharmaceuticals Inc. that reported early phase III data last month. (See BioWorld Today, Aug. 2, 2016.)
"We've considered what would be potential clinical trial designs that could accommodate that type of a scenario," Nolan said. "And that might range from sequential adaptive designs with active comparator to a scenario where you've got a single-arm, well-controlled trial."
Ultimately, the company will work with the FDA "to make sure we put forward and they're able to execute on the best possible trial design," he added, taking into account the trajectory of the disease and the company's existing data package. "What we want to do is get this therapy out as soon as possible and as safely as possible to kids suffering from SMA," Nolan said.
That explanation resonated with Jefferies analyst Biren Amin, who last month upgraded the company's stock to buy, from hold, revealing that feedback from an expert who participated in the nusinersen program suggested the FDA may allow for a single-arm pivotal trial of AVXS-101 in SMA type 1. The same expert opined that both SMA candidates offer "a compelling benefit," Amin wrote, "but to date, AVXS has delivered a better CHOP-INTEND benefit, in his view."
On Thursday, shares of Avexis closed at $36.01 for a gain of 60 cents.
