Checkpoint inhibitors (CIs) are among the hottest tickets in biopharma, with a handful of deals this year showing that no respectable oncology pipeline can be without one. Combined with traditional chemotherapy and radiation treatment, CIs made a big first impression in metastatic melanoma, thanks to the early success of Yervoy (ipilimumab, Bristol-Myers Squibb Co.). A checkpoint blocker against cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), Yervoy arrived on the market to great fanfare in 2011 and now is the centerpiece of numerous efforts to combine immunotherapies in cancer treatment, as documented in scores of abstracts over the past three years from the American Society of Clinical Oncology (ASCO), American Society of Hematology and other major medical meetings. (See BioWorld Today, March 28, 2011, June 7, 2011, May 16, 2013, Dec. 12, 2013, and May 15, 2014.)
A growing body of evidence suggests the targeted CI approach also has legs in non-small-cell lung cancer, leukemia, lymphoma and a variety of other tumor types – alone, in combination and, perhaps, even without the effects of systemic chemotherapy.
A review of immune-oncology combinations released this month by Thomson Reuters suggests the immuno-oncology (IO) segment is well on its way to becoming a multibillion-dollar market, with drugs like Yervoy and up-and-comer Opdivo (nivolumab) – so far approved only in Japan – leading the way. (See BioWorld Today, July 9, 2014.)
In fact, nivolumab is the first CI to advance to two phase III combination studies in melanoma, with Yervoy and Abraxane (nab-paclitaxel, Celgene Corp.), respectively, according to the report by Chaitali Talreja and Sonali Prusty, clinical research analysts at Thomson Reuters Recap, and Charlotte Jago and Alex Kibble, senior editors on the Thomson Reuters Cortellis Forecast team.
Combining monoclonal antibodies (MAbs) with small molecules is the most prevalent type of IO combination, followed by MAbs with vaccines, according to the analysis of clinical and research/deal-stage programs. Of 45 investigational IO combination drugs documented in the report, nearly half – 49 percent – comprise MAbs plus small molecules. Another 34 percent combine two MAbs; 13 percent combine MAbs and vaccines; 2 percent combine a MAb, small molecule and vaccine; and 2 percent combine cancer vaccines.
Because MAbs and small molecules have different manufacturing procedures and regulatory processes, "a company specialized in one may want to stick with that rather than breaking into something that would require new equipment, process and regulatory expertise," Jago told BioWorld Insight.
GROUND WAR HEATS UP WITH BATTLE OVER TARGETS
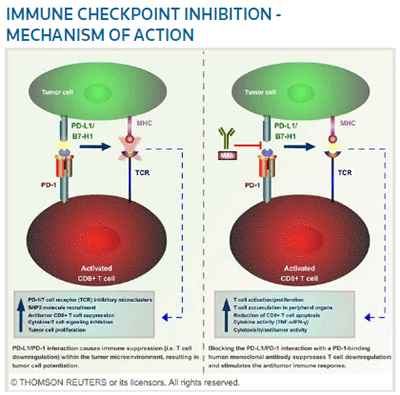 So far, biopharmas are battling over a very few targets, including CTLA-4, programmed cell death protein-1 (PD-1), and programmed death-ligand 1 (PD-L1), according to Jago. (See the Fig., right.) Opdivo, developed by Japan's Ono Pharmaceutical Co. Ltd. in collaboration with New York-based BMS to treat unresectable melanoma, was the first approved drug to target PD-1. In the U.S., BMS is pursuing a rolling biologics license application (BLA) for third-line pre-treated squamous cell non-small-cell lung cancer, expected to be completed by year-end, and a BLA for previously treated advanced melanoma.
So far, biopharmas are battling over a very few targets, including CTLA-4, programmed cell death protein-1 (PD-1), and programmed death-ligand 1 (PD-L1), according to Jago. (See the Fig., right.) Opdivo, developed by Japan's Ono Pharmaceutical Co. Ltd. in collaboration with New York-based BMS to treat unresectable melanoma, was the first approved drug to target PD-1. In the U.S., BMS is pursuing a rolling biologics license application (BLA) for third-line pre-treated squamous cell non-small-cell lung cancer, expected to be completed by year-end, and a BLA for previously treated advanced melanoma.
In September, BMS presented data from a pivotal trial of Opdivo at the European Society of Medical Oncology conference in Madrid suggesting the potential to treat BRAF wild-type melanoma without chemotherapy. Response rates to Opdivo were achieved in 32 percent of patients compared to 11 percent on chemotherapy. (See BioWorld Today, Sept. 30, 2014.)
Opdivo's moment in the spotlight was short-lived, however. In September, the FDA green-lighted Merck & Co. Inc.'s PD-1-targeting Keytruda (pembrolizumab) in melanoma, which gained the title of first U.S. drug approval in the class. The nod came almost two months before the drug's PDUFA date, although Whitehouse Station, N.J.-based Merck groused that restrictions in the label would cause dilemmas for prescribers and payers. (See BioWorld Today, May 7, 2014, Sept. 5, 2014, and Sept. 8, 2014.)
Pembrolizumab also is awaiting European approval for advanced melanoma, which could position it as the first European PD-1 inhibitor.
In the meantime, BMS is testing nivolumab in combination with Sutent (sunitinib, Pfizer Inc.) and Tarceva (erlotinib, Roche AG and Astellas Pharma Inc.), with data from both combos reported at the ASCO meeting in June. In May, BMS also inked a deal to test nivolumab in combination with Celldex Therapeutics Inc.'s CD27-targeting antibody varlilumab in a phase I/II study. (See BioWorld Today, May 15, 2014.)
Not to be outdone, in February Merck disclosed a trio of deals with Amgen Inc., of Thousand Oaks, Calif., Incyte Corp., of Wilmington, Del., and Pfizer to combine Keytruda with other cancer drugs. Terms were not disclosed in any of the alliances. (See BioWorld Today, Feb. 6, 2014.)
Another player emerging in the space is Astrazeneca plc, whose Medimmune Inc. unit is testing its CTLA-4-targeted antibody, tremelimumab, with PD-L1 candidate MEDI4736, which targets the B7 homolog 1, or B7-H1, and is also getting attention as a single agent. Medimmune has a separate deal pairing MEDI4736 with Incyte's oral indoleamine dioxygenase-1 (IDO) inhibitor, INCB24360. The IDO pathway regulates immune response by suppressing T-cell activation, which enables local tumor immune escape. (See BioWorld Today, May 17, 2013.)
And a largely unheralded component of a 2012 agreement between London-based Astrazeneca and Isis Pharmaceuticals Inc., of Carlsbad, Calif., is co-development of AZD9150 (ISIS-STAT3Rx), an antisense oligonucleotide inhibitor of STAT3, as an immunomodulatory agent in combination with MEDI4736. (See BioWorld Today, Dec. 12, 2012.)
DEALMAKING IN CIs APPEARS TO BE ACCELERATING
If the rush to CIs means that major oncology players need relevant assets in their pipelines, what's the effect on deal structures? One of the first major transactions in the space was a potential $713 million deal between Roche AG's Genentech Inc. subsidiary, of South San Francisco, and Array Biopharma Inc., which included $28 million up front and as much as $685 million in milestones, plus double-digit royalties, for rights to ChK-1 inhibitor ARRY-575 (GDC-0575), then in preclinical studies. Plans called for Genentech to develop the Array CI in parallel with its own GDC-0425 (RG7602) Chk-1 inhibitor before selecting one compound for further development. Genentech is currently evaluating the drug from Array, of Boulder, Colo., in a phase I safety study after completing a phase I study of its own asset, according to Cortellis Clinical Trials Intelligence. (See BioWorld Today, Aug. 10, 2011.)
The next significant agreement came a year ago, when Bayer Healthcare, of Whippany, N.J., inked a potential $540 million agreement with Compugen Ltd., of Tel Aviv, Israel. The collaboration, which included $10 million up front and another $30 million in preclinical milestones to Compugen in exchange for rights to two antibody-based therapeutics targeting immune checkpoint regulators, marked the first substantial revenue-sharing drug development alliance for Compugen and prompted a 44.5 percent run in its shares (NASDAQ:CGEN). (See BioWorld Today, Aug. 6, 2013.)
Dealmaking in CIs now appears to be accelerating. In March, Five Prime Therapeutics Inc., of South San Francisco, received $20 million up front and sold a premium-priced equity stake for another $21 million as part of a three-year cancer immunotherapy deal with BMS centered on two undisclosed pathways that could each generate several targets. BMS pledged up to $300 million per target in milestone payments related to development, regulatory and sales goals. (See BioWorld Today, March 18, 2014.)
Two CI deals were disclosed in April. Sentinel Oncology Ltd., of Cambridge, UK, could earn up to $174 million from an alliance with Oncothyreon Inc., of Seattle, on its preclinical drug development program targeting checkpoint kinase 1 (Chk1). The companies did not disclose details about the deal structure, although Sentinel revealed the arrangement was not back-end loaded. (See BioWorld Today, April 23, 2014.)
And Agenus Inc., of Lexington, Mass., inked a collaboration and license agreement with Merck to discover and optimize fully human antibodies against two undisclosed checkpoint targets using its 4-Antibody Retrocyte Display platform. Agenus could receive about $100 million in clinical, regulatory and commercial milestone payments for the candidates as well as product royalties. Agenus gained rights to the platform technology through its acquisition of European biopharma firm 4-Antibody AG, in February.
The IDO pathway was the focus of the latest large CI deal between Genentech and Newlink Genetics Corp., of Ames, Iowa, which could exceed $1 billion as the companies develop NLG919, Newlink's indoleamine 2,3-dioxygenase pathway inhibitor, and embark on a broader research collaboration to discover next-generation IDO/tryptophan 2,3-dioxygenase, or TDO, compounds. Newlink received an up-front payment of $150 million in that agreement, signed last month. (See BioWorld Today, Oct. 21, 2014.)
'PEOPLE ARE LESS AFRAID OF NEW STUFF'
Despite the hurry for pharmas and big biotechs to option or outright acquire CI assets, Laura Vitez, senior deals analyst for Thomson Reuters Recap, said recent deals are fairly conventional, mostly with "pretty straightforward early stage licenses." The Agenus and Five Prime deals both featured assets still in discovery, while Array and Sentinel were preclinical and Newlink's asset was in phase I. All were global arrangements. Partnering at those stages is not unlike trends in other therapeutic categories, where "very generally, dealmaking has been really strong at the early stages over the past year to year and a half," Vitez told BioWorld Insight.
"It's really wonderful to see a lot of deal activity in innovative science, or what some people would call 'high' science," she added. "People are less afraid of new stuff than they were during the dark days" of the Great Recession. Admittedly, companies also may be compelled to do earlier-stage CI deals since many of those assets were forced into dormancy when biotech investment collapsed during the recent recession, Vitez pointed out.
"If you want to stuff your pipeline, you have no other choice," she said.
That said, it's instructive to look at targets involved in each deal to determine whether they complement or compete with the seller's internal pipeline, according to Vitez. For instance, since Genentech has suggested it may test several compounds, in addition to ARRY-575, in combination with GDC-0425, it makes sense that Array may be set to receive royalties on a commercial product either way, or a return of its asset, if the partnership falls through, she suggested. Otherwise, "why would Array license to Genentech only to see its drug killed?" she asked. (See BioWorld Today, April 10, 2013.)
Previous transactions between Biogen Idec Inc. and Isis and between Astrazeneca and Abgenix Inc. had related structures, "so there's some precedent," Vitez said. (See BioWorld Today, Oct. 17, 2003, Jan. 1, 2012, and July 2, 2012.)
In any event, don't expect to see thinning in the CI traffic any time soon. In an update on the IO market published In July, Leerink Partners LLC biotech analysts Seamus Fernandez, Howard Liang and Michael Schmidt increased their peak sales forecast for the space to $36 billion, from $32 billion in May and $29 billion in November 2013, citing "increased conviction in the clinical utility of PD-1/PD-L1 antibodies across a broad range of solid and liquid tumors." With data continuing to emerge on new tumors and new PD-1/PD-L1 combinations, "the only constant is likely to be change," they added.
