If you look at the chart (see below) of the number of biotech initial public offerings (IPOs) that have been completed annually in the U.S. during the past 15 years, one year stands out above all others – 2000. The 66 IPOs that were concluded that year represents a lasting testimony to the euphoria around genomics and information technology at the time that catalyzed investors to eagerly snap up new offerings in companies focused on biotechnology and drug development.
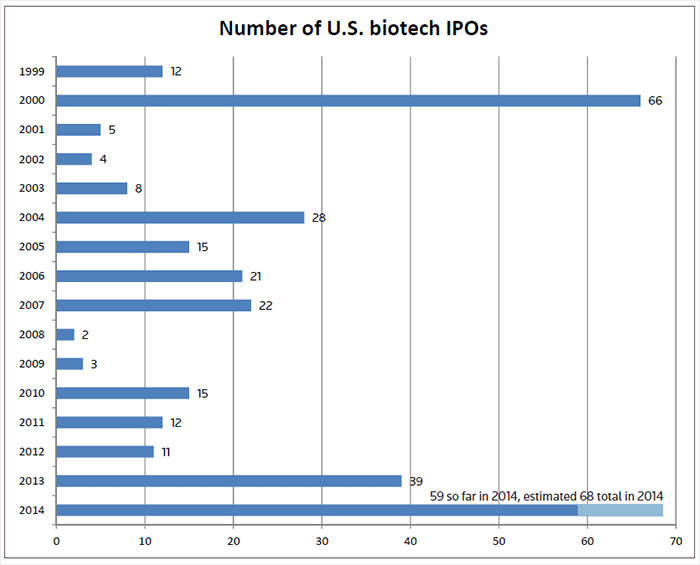
It was thought that this record number of IPOs would never be broken given the challenging capital markets that have ensued since then. But that idea is one that is about to be dispelled. With just over one quarter of the year remaining, there have already been 59 U.S. biotech IPOs, including three that priced last week, with positive reception from investors.
Proqr Therapeutics BV, of Leiden, the Netherlands, for example, offered 7.5 million shares (upsized from 6.3 million) at $13 each, which was at the top end its planned $11 to $13 range; the underwriters were granted a 30-day option to purchase up to 1.125 million additional shares. In addition, prior to pulling the trigger on its offering, the company filed an F-1 with the SEC to register approximately 1.44 million additional shares, pushing the firm’s potential gross proceeds past $112 million. The company’s shares (NASDAQ:PRQR) opened above the selling price and closed the day at $14.73 up 13 percent. (See BioWorld Today, Sept. 19, 2014.)
Proqr plans to use IPO proceeds to advance the preclinical cystic fibrosis (CF) candidate QR-010 through phase IIa trials. The technology was licensed from Massachusetts General Hospital, and the company said it believes the compound can become a disease-modifying therapy in CF patients with the DF508 mutation, which affects about 70 percent of the total CF population. The company plans to file an investigational new drug application in the U.S. and a clinical trial authorization in Europe later this year.
ENTHUSIASM
Enjoying a similar enthusiastic reception was Tokai Pharmaceuticals Inc., of Cambridge Mass., which priced its IPO of 6.48 million shares at $15 each on Wednesday for proceeds of $97.2 million, a day after filing an amended S-1 to register up to $18.63 million in additional shares. The company, which initially sought to raise $75 million, priced the IPO at the top of the proposed range and granted underwriters a 30-day option to purchase up to 972,000 additional shares. The company’s shares (NASDAQ:TKAI) opened at $18.63 and rocketed to $30 before easing back to $23.76 for a gain of $8.76, or 58.4 percent, on volume of 10.4 million.
Proceeds from the transaction will be used to advance lead compound galeterone, a selective, multitargeted, oral small-molecule prostate cancer drug in phase II trials in castrate-resistant prostate cancer (CRPC). It is expected to enter pivotal trials in the first half of next year.
The androgen receptor signaling pathway – the primary pathway driving prostate cancer growth – ordinarily is activated by the binding of male hormones, or androgens, such as testosterone and the more potent androgen dihydrotestosterone (DHT) to the ligand-binding domain of androgen receptors in prostate cancer cells. Galeterone disrupts the activation of that pathway using multiple mechanisms: inhibiting the enzyme CYP17, which blocks the synthesis of testosterone; provoking androgen receptor antagonism, which blocks the binding of testosterone or DHT with the androgen receptor; and degrading the androgen receptor, reducing the amount of androgen receptor protein in the tumor cells.
Tokai is seeking to prove that galeterone’s combined mechanisms of action may differentiate the drug in CRPC, providing efficacy advantages over therapies that act solely through CYP17 inhibition or androgen receptor antagonism, reducing the risk of or delaying the development of resistance to therapy and providing efficacy in patients with tumors resistant to other treatments. (See BioWorld Today, Aug. 13, 2014.)
Although Foamix Pharmaceuticals Ltd., of Rehovot, Israel, priced its IPO at just $6, after setting an initial target range of $10 to $12, the firm raised $40.2 million, and the company’s shares (NASDAQ:FOMX) opened at $8 and traded up to $11 before they settled back toward earth to close at $6.22, or a gain of 22 cents, on opening day. Nearly 16 million shares changed hands, reflecting the growing investor interest in the antibiotics field.
Foamix said it plans to move its lead candidate, FMX101, a minocycline foam formulation, into phase III and conduct other pre-launch studies needed to file for approval in moderate to severe acne.
THE MAGIC NUMBER IS EIGHT
Excluding the five European IPOs conducted so far this year, the number of biotech IPOs completed in the U.S has reached a staggering 59 and there is no reason to believe that we won’t see a further eight IPOs getting done before the year draws to a close. In fact, we estimate that when the dust settles a new high water mark will be set at 68 transactions.
We are certainly not alone in our bullish prediction. Renaissance Capital, a global IPO investment adviser, in its “Fall 2014 US IPO Preview” said 188 companies have raised $40 billion so far, a 44 percent increase in deal count year over year. “This high level of activity looks set to continue as a growing backlog suggests that up to 100 more IPOs could raise as much as $40 billion. That would bring 2014’s total proceeds to $80 billion, up 46 percent over 2013 and the most proceeds raised since 2000.”
BioWorld Snapshots lists 29 companies that have filed for IPOs and just last week Neuroderm Ltd., which is seeking to raise $65 million to advance a pipeline of drugs targeting Parkinson’s disease and other central nervous system disorders, added its name to the biotech IPO runway.
The increased number of biotech offerings is likely to continue into the fourth quarter, the Renaissance report noted, “due to positive clinical trial results and recent M&A activity.” The large anticipated pipeline also will allow investors to be increasingly picky. Several biotechs we list below boast collaborative funding agreements, well-known backers and multiple drug candidates with large addressable markets.
Among the runway lineup all eyes will be on Forward Pharma A/S, a Danish biotech developing a dimethyl fumarate (DMF) therapy for immune disorders, The company is hoping to generate $200 million from an IPO, an amount that is rarely attained by biotech IPOs since several companies broke that mark in 2000.
The company plans to direct about $80 million of the offering’s net proceeds to pursue a phase III trial of FP187, its DMF therapy for the treatment of relapsing remitting multiple sclerosis, with the goal of supporting a new drug application in the U.S. and a European marketing authorization application. Another $22 million will be dedicated to supporting development of FP187 for the treatment of psoriasis, a program in which the company plans to initiate a phase III study by early 2015. Preclinical programs and phase I trials would be allotted about $12 million. (See BioWorld Today, Aug. 12, 2014.)
BY THE NUMBERS
As a group, the 2014 IPO graduates continue to perform well with their share values growing by a collective average of 10 percent year-to-date. Among the leading gainers is Coppell, Texas-based ZS Pharma Inc., which filed in May as an emerging growth company looking to raise $86 million, ended up pricing its offering $1 higher than its proposed range at $18. It also increased the number of shares offered – from 5 million to 5.9 million – to bring in gross proceeds of $107 million. Its shares (NASDAQ:ZSPH), which closed up 57.7 percent on its first day of trading, had gained 103 percent from its IPO price as of Thursday.
 The company has a lead product, ZS-9, indicated for hyperkalemia, a condition for which few options are available, in phase III trials.
The company has a lead product, ZS-9, indicated for hyperkalemia, a condition for which few options are available, in phase III trials.
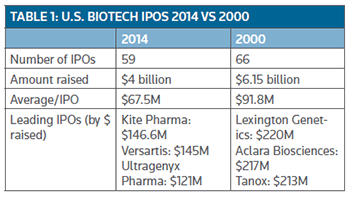
Table 1 provides an interesting comparison on the class of 2000 IPO companies with the current crop of 2014 graduates to date. One striking features is the whopping $6.1 billion raised by the 66 companies compared with the $4 billion generated so far by 59 companies.
