There is no doubt that the close to 16,000 registered delegates attending the 2014 BIO International Convention in San Diego a couple of weeks ago returned home in positive mood. No surprise really because by any partnering or financial metric the statistics are pointing to the fact that biotechnology is in really good shape at the halfway point of the year.
After a brief two-month slide from its high point at the end of February, the BioWorld Biotech Blue Chip index, a price-weighted index that includes 20 of the top biotech companies by market cap, has recovered almost all the value it lost during that period. (See BioWorld Blue Chip Index, below.)
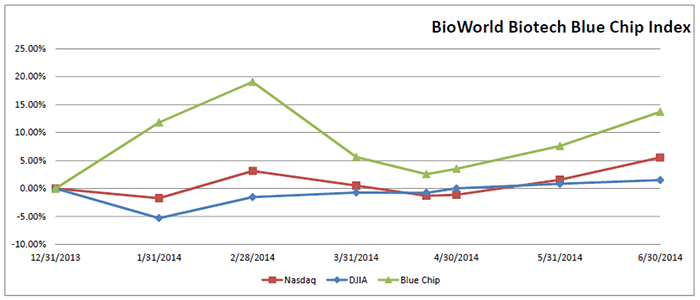
The Index closed out the month of June on a high, increasing 5.7 percent and outpacing the Nasdaq Composite index and Dow Jones Industrial average, which saw their values grow by 3.6 percent and 0.65 percent, respectively.
Top gainers in the group included Vertex Pharmaceuticals Inc., whose shares surged on strong phase III cystic fibrosis data for a combination regimen of its VX-809 (lumacaftor) and Kalydeco (ivacaftor), which offered marked improvements in lung function and hit multiple secondary endpoints. (See BioWorld Today, June 25, 2014.)
Vertex plans to submit regulatory filings in the U.S. and Europe in the fourth quarter of this year, and given a green light, a product launch could come as early as the second half of 2015. According the Wells Fargo analyst estimates, they expect the drug combo to be priced around $166,000 per year in the U.S. upon launch; that would lead to blockbuster sales of $4.3 billion by 2021. The company's stock (NASDAQ:VRTX) value closed out the month at $94.68, up 31 percent.
Shares in Dublin-based Shire plc closed June up almost 36 percent as the company became an acquisition target of Abbvie Inc., which is offering $46 billion in cash and shares for the specialty pharma and rare diseases company.
Another top gainer was Isis Pharmaceuticals Inc., whose shares (NASDAQ:ISIS) closed up 18 percent on the strength of favorable phase II data showing that the company's experimental antisense drug, ISIS-GCGRRx, helped patients with type 2 diabetes improve control of the disease, even when they're unable to do so with metformin. In addition, the Carlsbad, Calif.-based company reported it had earned a $1 million milestone payment from Glaxosmithkline plc related to the advancement of a phase II/III study of ISIS-TTRRx in patients with familial amyloid polyneuropathy.
Celgene Corp. completed a 2-for-1 stock split in the month, increasing the total number of shares outstanding from approximately 399 million to approximately 798 million. The company's shares closed up 12 percent for the month.
During the second quarter, the BioWorld Blue Chip Index increased 7.6 percent in value and has gained 13.7 percent in value year to date.
CREDIBLE GROWTH
The BioWorld Growth Index, which includes companies with market caps in the range of $1 billion to $3 billion and a résumé that typically includes a strong drug pipeline and partnered products in late-stage clinical trials, saw its value grow by a credible 9.4 percent in June. However, it still has a ways to go before it gets back to the high point reached in February; share prices of companies in that group can pivot quickly based on the fortunes of their lead products. (See BioWorld Growth Index, below.)
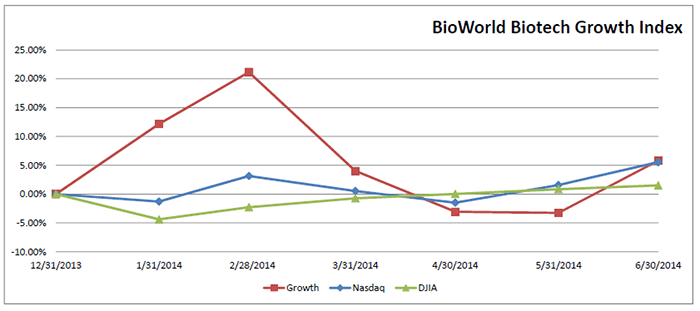
Rare disease specialist Synageva Biopharma Corp. is a typical example. The company was the leading gainer with its share value jumping 29 percent in June. Shortly after close of the quarter, it reported top-line phase III data showing that sebelipase alfa (SBC-102), its orphan-designated enzyme replacement therapy for lysosomal acid lipase (LAL) deficiency, met its primary and several secondary endpoints. The results showcased the therapy's impact on multiple symptoms of LAL deficiency and set Synageva up to support FDA and EMA registration applications for the therapy by the end of the first quarter of 2015. Despite the positive news, Synageva's shares (NASDAQ:GEVA) tumbled more than 13 percent to $90.42 July 1, possibly due to concerns about a single treated patient who withdrew from the study due to an infusion-related reaction. (See BioWorld Today, July 2, 2014.)
Mannkind Corp. also had good news to impart to its investors, revealing that its inhaled insulin Afrezza (insulin human [rDNA origin]) finally won FDA approval. The company's shares (NASDAQ:MNKD) closed the month up 23 percent.
More good news drove up the share value of San Diego-based Halozyme Therapeutics Inc. (NASDAQ:HALO) to close the month at $9.88, a 25 percent jump. The FDA removed the clinical hold on patient enrollment and dosing of PEGPH20 in the company's ongoing phase II trial (Study 202) in pancreatic cancer, permitting the study to resume under a revised protocol.
Not all companies in the group posted increases in their share prices. Clovis Oncology Inc. (NASDAQ:CLVS) posted a drop of 19 percent in June. What spooked investors was the reported elevated glucose levels emerging in roughly one-fourth of non-small-cell lung cancer patients receiving the company's next-generation oral EGFR inhibitor CO-1686. Those data overshadowed the promising interim progression-free survival (PFS) rate presented at the American Society of Clinical Oncology meeting last month. (See BioWorld Today, June 3, 2014.)
RISING FORTUNES
Typifying the fact that the rising fortunes of the biotech sector are not just being driven by its larger companies was the 25 percent hike in the value of the BioWorld Emerging Biotech Index. (See BioWorld Emerging Biotech Index, below.)

"Investors have been quick to reward several smaller-cap stocks for early stage clinical progress," noted Cowen and Co. analysts in their Biotechnology Thermometer report.
Helping drive the index was the whopping 284 percent leap in Idenix Pharmaceuticals Inc.'s share price (NASDAQ:IDIX) following the announcement that Merck & Co. Inc. would be acquiring the company for $24.50 in cash per share, or about $3.85 billion, in a 300 percent-plus premium deal approved by the boards of both companies and set to close during the third quarter of this year. (See BioWorld Today, June 3, 2014.)
With the transaction, Merck, of Whitehouse Station, N.J., gains access to Idenix's IDX21437, a uridine nucleotide (nuc) analogue HCV NS5B polymerase inhibitor for which the firm recently reported data from a phase I/II trial. During the seven-day proof-of-concept part of the study, IDX21437 yielded mean maximum 4.2 .og10 IU/mL to 4.3 log10 IU/mL reductions for patients infected with HCV genotype 1, 2 or 3 receiving 300 mg once daily.
Also boosting the BioWorld Emerging Biotech Index was a 52 percent increase in the share value of Insmed Inc. in June. Investors jumped on board following the company's announcement that its lead compound Arikayce (inhaled liposomal amikacin) achieved FDA breakthrough designation in nontuberculous mycobacterial lung disease, despite missing its primary endpoint in a phase II trial in that indication. The company plans to work with the FDA to determine the optimal pathway to a possible regulatory approval for Arikayce. (See BioWorld Today, June 19, 2014.)
Our Indices are clearly indicating that the prospects for the sector will remain bullish, certainly well into the third quarter and possibly beyond. Although we are unlikely to see the huge gains posted during the 14-month period from January 2013 there will be steady advances sustained by strong upcoming second quarter financial results.
