BB&T Contributing Editor
WAILEA MAUI, Hawaii — The annual Hawaiian Eye Meeting was held here in January at the Grand Wailea Resort as specialists from three key ophthalmic specialties – cataract, refractive and retinal surgery – gathered to hear the latest clinical information and to enjoy the sunny and warm weather.
Despite the decline in the income in the refractive surgery community due to the plunge in their lucrative LASIK procedures, physician attendance of about 850 was at a record level. It appeared that increased attendance was accounted for partially by the retinal specialists. Attendance from the ophthalmic industry was definitely lower.
The retinal sessions were heavily dominated by discussions on current and potential future treatments for age-related macular degeneration (AMD). As its name implies, AMD is associated with aging, afflicting 18% of those between 70 and 74 years old, surging to 47% among people 85 years and older. It is a chronic, progressive disease of the macula, the central part of the retina, causing irreversible loss of central vision. AMD is the No. 1 cause of severe vision loss and blindness among those over age 50 in the developed world and currently affects the vision of more than 2 million Americans of ages 50 and older.
But despite the severe damage AMD can cause, the public is still relatively unfamiliar with the disease. Indeed, a survey conducted by AMD Alliance International (Toronto) in 2008 found that more than half of all respondents had either never heard of AMD, or had heard of it but knew very little about it.
The key risk factors for AMD, as provided by the National Eye Institute, are shown in Table 1.

Prior to 2000, there were essentially no effective treatment options for halting or reversing the course of AMD, which results in a big decline in an elderly person's quality of life and mental well-being.
Several studies have found that there is a higher rate – as much as two to five times greater – of clinical depression among elderly patients with impaired vision, when compared with similarly aged patients without sight impairments. Since 2000, three new pharmaceutical agents have reached the U.S. market, producing vast improvement in patient outcomes.
The FDA approval in mid-2006 of the Genentech (South San Francisco, California)-developed drug Lucentis literally revolutionized the treatment of AMD because this agent cannot only halt the progression of the disease, but in many patients it has miraculously improved their vision.
Lucentis inhibits vascular endotlhelial growth factor (VEGF), an important signaling protein involved in angiogenesis, the growth of blood vessels from pre-existing vasculature. In AMD, it is the growth of these new blood vessels, which called neovascularization, that is a major culprit in vision loss.
Lucentis has been a tremendous commercial success in U.S., with its sales in 2008 estimated at about $875 million. Its marketing partner, Novartis Pharma (Basel, Switzerland), recently reported calendar 2008 sales of $886 million, a robust 122% gain over the previous year. Novartis holds the marketing rights for all countries globally except the U.S.
Despite its impact on AMD, Lucentis remains a very controversial drug, as its huge expense—approximately $2,000 for the drug itself and another $600 in associated office visit costs per injection — and the need to inject it frequently has been a burden to the budgets of seniors, ophthalmologists and to Medicare.
Avastin, the "mother molecule" of Lucentis, is an FDA-approved anti-VEGF drug which generated about $2.7 billion of global revenue in 2008, almost all from the oncology market. Avastin is not FDA-approved for AMD, but because it is chemically very similar to Lucentis, is far cheaper (about $50 per dose when used to treat AMD) and is easily extracted for ophthalmic use, its off-label use for AMD has surged in recent years.
The exact amount of ophthalmic Avastin usage is not known but it is clearly significant. During a session on the treatment of advanced (wet) AMD, moderator Carmen Puliafito, MD, dean at the Keck School of Medicine at the University of Southern California (Los Angeles), posed the question to the audience: "what is your preferred anti-VEGF drug?" Avastin was the preferred choice by 59% of the responders vs. 38% for Lucentis. Similarly, 35% of the physicians were using Avastin exclusively, compared to only 15% for Lucentis.
In a talk on anti-VEFG agents, Jay Duker, MD, chairman of the department of ophthalmology at Tufts New England School of Medicine (Boston), said "there are lots of unanswered questions" for the treatment of wet AMD and that "the biggest question is the issue of Lucentis versus Avastin."
The Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) sponsored by the National Eye Institute of the National Institutes of Health was launched about two years ago to resolve the question of which drug is safer and more effective. CATT will enroll 1,200 patients with newly-diagnosed wet AMD.
Secondary endpoints for this trial are how frequently the drugs should be administered and the comparative cost differential of these treatments. At approximately 20 times the cost of Avastin, it will be necessary for the Lucentis safety and efficacy data to just this massive price premium.
The results of the CATT, which are eagerly awaited, are not expected to be available for at least two more years.
A small pilot trial comparing the two drugs head-to-head was presented here by Manju Subramanian, MD, assistant professor of ophthalmology at Boston University School of Medicine. Her 20-patient trial with three-month follow-up showed a preliminary trend that Avastin patients fared better in vision improvement, though diagnostic measures – such as optical coherence tomography – of the health of the retina showed that Lucentis performed somewhat better.
Noting that while the trial "is not statistically significant because of the small number of patients and limited follow-up," she indicated that more data would be forthcoming in the coming months.
Previous surveys of retinal practices has shown that on average Lucentis or Avastin is injected five or six times a year. This was corroborated by Anne Fung, MD, clinical professor at California Pacific Medical Center (San Francisco), who presented data from a 129-patient registry which began more than two years ago. It showed that an average of 5.4 anti-VEGF injections were used annually.
Ionizing radiation seen as potential solution
The significant cost of anti-VEGF therapy and the requirement that patients be seen very regularly imposes, in the words of Puliafito, "a therapeutic burden" for physicians and their patients. One potential and promising solution to this dilemma appears to be the use of ionizing radiation.
Elias Reichel, MD, director of the New England Eye Center (Boston), said that radiation has several positive attributes – it is anti-angiogenic, anti-inflammatory and anti-fibrotic – and that "there would be a significant benefit if we could reduce the number of anti-VEGF injections."
Two privately-owned, venture capital-backed companies – NeoVista (Fremont, California) and Oraya Therapeutics (Newark, California) – are working in this area.
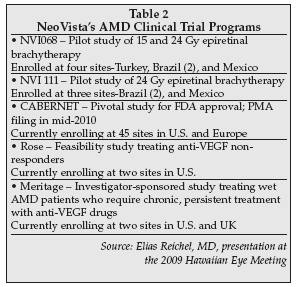
NeoVista has developed the Epi-Rad90 Ophthalmic System, in which a surgeon delivers 24 Gy of focal strontium-90 beta radiation directly to the macula through a small incision in the outer layer of the eye. This approach is often referred to as epiretinal brachytherapy.
Unlike external beam radiation, this novel system ensures delivery of a therapeutic dose of beta radiation to the wet AMD lesion and avoids any deleterious radiation exposure to other ocular structures such as the optic nerve or the crystalline lens.
Its two feasibility trials have demonstrated no toxicity to surrounding ocular tissues and the efficacy data was solid, with 73% of subjects requiring no additional anti-VEGF injections.
The company is currently enrolling patients in its pivotal trial, dubbed the CNV Secondary to AMD Treated with Beta Radiation Epiretinal Therapy (CABERNET). The trial is being conducted at roughly 40 sites worldwide (20 in the U.S.) and is randomizing patients to either strontium-90 plus Lucentis or to Lucentis alone.
A total of 450 patients will enter the trial, 300 in the NeoVista arm and 150 in the Lucentis only arm. The primary endpoint is to determine which regimen will enable patients' vision to either improve the most or deteriorate the least. Enrollment is expected to be completed by mid-2009 and a PMA filing, after a required one-year follow-up, is expected to occur by mid-2010.
Two other NeoVista studies are underway: ROSE, a feasibility study to determine the safety and efficacy of radiation therapy in those who do not have optimal response to treatment with anti-VEGF medication alone and the Macular EpiRetinal Brachytherapy In Treated Age Related Macular Degeneration Patients (MERITAGE) feasibility trial. The latter is now enrolling patients to will evaluate radiation therapy in wet AMD patients who require frequent persistent chronic therapy with anti-VEGF agents.
A listing of all of NeoVista's trials in provided in Table 2 on page 19.
Oraya's IRay System is designed to deliver a highly localized dose of low-energy X-Ray radiation non-invasively to the macula using a robotic positioning system, targeting algorithm and a device for eye stabilization. Its procedure can be performed quickly in the doctor's office under a topical anesthetic.
Oraya is currently enrolling patients into its feasibility trial outside the U.S., and some of its six-month data was presented at this meeting. The device has performed well with the impressive initial safety and efficacy data. A U.S. and European trial is expected to commence in the near future.
Commenting on the clinical progress from the two companies, Puliafito proclaimed that a combination approach using ionizing radiation and anti-VEGF agents is "very exciting and bears careful evaluation."
Another approach to advancing the treatment of advanced AMD may be combination drug therapy. This approach involves the use Lucentis or Avastin in combination with photodynamic therapy (Visudyne) alone or the addition of a generic steroid (typically either dexamethasone or tramcinolone.
Positive six-month results of a randomized "triple therapy" clinical trial were reported here by Henry Hudson, MD, from the Retina Center (Tucson, Arizona), The study showed that the triple therapy group – in this case Lucentis, Visudyne and dexamethasone – showed the most gain in visual acuity when compared with monotherapy and double therapy. In addition, patients receiving monotherapy required about 3.5 treatments while combination therapy treatments, both triple and double, averaged two treatments.
Hudson, said, "the cumulative re-treatment rates were lower in the combination group but it is a little early to determine what this final number is going to be."
According to the National Institutes of Health Clinical Trials Registry, at least 17 studies are under way to compare some form of combination treatment with the gold standard of Lucentis sole therapy.
It is clear that combining different agents has become a widespread practice in the retinal world, especially in Europe where several trial shave been completed. In an article in the Feb. 1 European/Asia Pacific edition of Ocular Surgery News, titled "AMD Treatment in Europe Focuses on Combination Therapies, New Innovations," Paolo Lanzetta, MD, of the department of ophthalmology at the University of Udine (Udine, Italy), said that "different agents target different pathways and together (they) might have a synergistic effect."
Premium IOLS show strong growth
The severe downturn in the economy has had a major impact on many consumer discretionary medical procedures, including laser refractive surgery (LASIK). The widely-quoted industry leader in ophthalmic surgery market research, MarketScope (St. Louis), has reported that domestic LASIK procedures declined about 23% to about 1.0 million procedures in 2008 compared to 2007, with the lion's share of the decrease occurring in the last few months of 2008. For 2009, its forecast is equally dismal, with a decline of over 25% to about 750,000 procedures.
In the midst of this terrible LASIK showing, it is interesting to note that the market for premium intraocular (P-IOLs) continued to register solid growth throughout 2008. The category of P-IOLs includes multifocals, accommodating, toric, aspheric, blue-blocking and micro-incision IOLs, all of which are premium-priced relative to a simple monofocal IOL, which typically sells for about roughly $100 per lens in the U.S.
According to a recent article in "Ophthalmic Market Perspectives," published by MarketScope, global IOL revenue grew about 10% in 2008, reaching over $1.9 billion, with the robust growth of P-IOLs fueling the growth.
Presbyopia-correcting IOLs (PC-IOLs) have been a major contributor to the overall growth of the premium IOL market. Based on comments from physicians and industry sources, Biomedical Business & Technology believes that demand for these lenses has continued to increase, despite the downturn in the economy and the fact that there is a significant out-of-pocket expense – perhaps $2,000 to $2,500 per eye – for the patients.
In order to better understand this market, SM2 Strategic (Pleasanton, California) recently conducted a consumer survey, which was paid for by Bausch & Lomb (B&L; Rochester, New York). B&L sells the hugely successful Eyeonics Crystalens HD accommodating IOL. The goal of the survey was to understand consumer price sensitivity and provide data on consumer attitudes towards the purchase of a premium-priced presbyopia-correcting IOL.
Amongst several findings, 54% of all patients interviewed said that they would pay more for the "all distance" vision provided by Crystalens HD, with nearly 28% saying that "money is no object as far as my eyes are concerned."
The two main conclusions from the survey were: 1) There is clear demand and willingness to pay for a premium IOL and 2) Functional vision at all distances is the key appealing reason.
Several speakers here addressed the PC-IOL topic during the week here, some extolling the virtues of their favorite lens, others discussing tactics to grow this business in the face of a rugged economic climate. Ralph Chu, MD, of Chu Vision Institute (Edina, Minnesota), speaking at a B&L-sponsored meeting, said that his preferred PC-IOL is the Eyeonics Crystalens HD because it "mimics the natural focusing action of the eye" and that it provides improved near vision compared to earlier models of the Crystalens.
Very positive comments on Alcon's (Fort Worth, Texas) ReSTOR 3.0 multifocal IOL were expressed by Kerry Solomon, MD, of Medical University of South Carolina's Storm Eye Institute (Charleston, South Carolina). This lens, which was approved in the U.S. in late December, is now entering the market with strong support from many cataract/refractive physicians. It will likely quickly supplant the previous-generation ReSTOR 4.0 lens as the PC-IOL of choice for many physicians.
According to Solomon, the several design changes implemented from the previous ReSTOR 4.0 model have resulted in better near reading and intermediate (computer) distance visual acuity, thereby "providing another option for surgeons and patients."
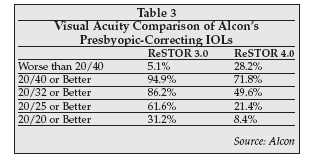
At a presentation at the JP Morgan Health Care Conference in January in San Francisco, Alcon CEO Carey Rayment was very enthusiastic about the potential for the ReSTOR 3..0 lens, citing several positive following attributes: 1) It maintains all visual performance benefits of the 4.0 at near, intermediate and distance; 2) It extends reading distance by 7 cm (2.8"); 3) It improves intermediate visual acuity by 1 to 1.5 lines; 4) Its aspheric optic provides improved image quality; 5) It provides a much higher rate of patient satisfaction and spectacle independence. Rayment's comparison of the two ReSTOR lenses is contained in Table 3.
Competition in the PC-IOL sector has clearly heated up in the past several months, with FDA approvals for improved products from B&L, Alcon and most recently, Advanced Medical Optics (AMO; Santa Ana, California). After a lengthy delay, the latter received FDA clearance for its Tecnis multifocal IOL (MF-IOL) in mid-January.
The Tecnis MF-IOL will likely quickly obsolete AMO's other approved PC-IOL ReZoom, which has been suffered a decline in its popularity in the U.S. in 2008, heavily related to its poor performance in the near field and the impact from the mid-2008 launch of Crystalens HD.
The unique design of this lens, which has been well-received in Europe, offers patients improved near, intermediate and far distances in both day and night, with nearly nine out of 10 patients reporting that they never wear glasses after surgery. According to AMO, it has a 95% patient satisfaction, the highest of any presbyopia-correcting IOL.
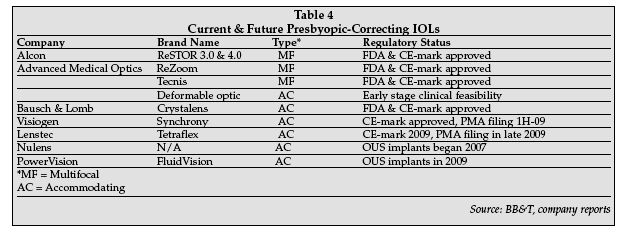
More PC-IOLs are in development and Table 4 on page 20 provides information on currently approved products and potential future entrants into this market. There is no doubt, based on the aging of the population and the apparently strong desire for patients needing a cataract surgery to opt for a more costly PC-IOL, that this market segment will enjoy strong growth in the next several years.