BBI Contributing Editor
LOS ANGELES The American Society of Breast Surgeons (ASBS; Laurel, Maryland), founded only as recently as 1995 and representing more than 2,000 surgeons, is one of the fastest-growing subspecialty surgical groups in the country and is the largest group of physicians specifically devoted to the advancement of surgical treatments and techniques for the treatment of breast disease. This group of elite surgeons, who dedicate at least 50% of their surgical practice to breast disease patients, upholds a mantra of "finding the most effective way of eliminating all cancer cells while maintaining the best possible cosmetic effect." In other words, they are attempting to transform the old-fashioned approach of treating breast cancer from the "maximum tolerated treatment" to the "minimum effective treatment."
The ASBS annual meeting, held here in mid-March, offered the latest scientific data and clinical research on diagnostics and therapies, discussions on controversial medical and ethical issues, and participation in courses on the newest procedures intended to improve the quality of care for the more than 1 million patients who undergo breast surgery each year in the U.S.
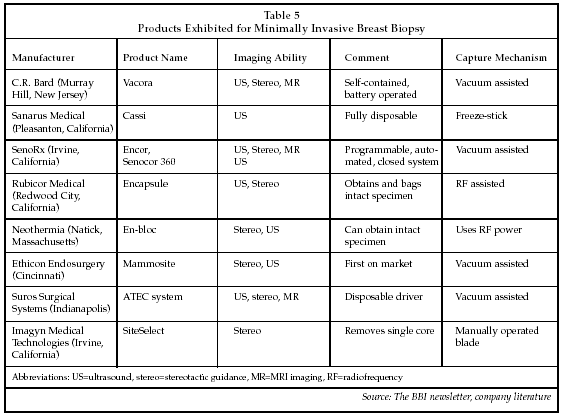
With more and more evidence that minimally invasive surgical excision of breast cancers (lumpectomies) is as effective as mastectomy, the next natural step for treatment of these patients is also to find the minimum radiation dosage effective for complete destruction of cancer cells equivalent to traditional external beam radiation but without the side effects and duration. Accelerated partial breast irradiation (APBI) is a relatively new procedure to most surgeons and radiation oncologists in which the portion of the breast at highest risk of recurrence receives a shortened course of high-dose radiation therapy (RT). Several techniques that can deliver the RT over a shorter period of time are being investigated, including single-dose intraoperative, localized external beam, and balloon catheter inserted into the lumpectomy cavity. The ASBS feels that these techniques should all be performed under an approved protocol as part of a clinical study and that patient selection criteria should be: age over 50, total tumor size (invasive and ductal carcinoma in situ) less than or equal to 2 cm in size, negative margins of at least 2 mm, and lymph node negative. About one-third of the 267,000 new breast cancer patients in the U.S. annually would meet these criteria. Four of these new techniques were being shown on the exhibit floor (see Table 4).
Proxima Therapeutics (Alpharetta, Georgia), which was acquired in March by Cytyc (Marlborough, Massachusetts), was the first to enter the breast brachy-therapy market, utilizing a balloon that is inserted into the lumpectomy cavity following surgery. Radioactive isotopes are then delivered into the balloon to radiate the lumpectomy cavity, the most likely area for recurrence. This method of radiation therapy reduces the typical twice a day for 30 days regimen down to five days twice a day. Good/excellent cosmetic results were reported in more than 88% of patients at two years after treatment and local recurrence rates have been 0% at two years of follow-up.
Electronic brachytherapy, a new modality for accelerated partial breast irradiation, was being exhibited by Xoft (Fremont, California) for the first time. Following the same concept as Proxima, a balloon is inserted into the lumpectomy cavity and then a miniaturized X-ray source delivers radiation to the tumor bed. The biggest difference is that it delivers electronic radiation instead of radioactive radiation. This means that the procedure does not need to be performed in radiation bunkers which have walls that are lead shielded or are made of very thick concrete. Instead, brachytherapy can be delivered in regular treatment rooms. Xoft's 510(k) is in process and, after clearance, it expects to have 40 patients treated in an initial clinical study this summer followed by a 1,300 patient registry.
Carl Zeiss (Oberkochen, Germany) exhibited its Intrabeam Intraoperative Radiation Therapy (IORT) that delivers low-energy, high-dosage radiation through a rounded size-specific probe to the tumor bed immediately after excision. Because the Intrabeam uses very low energy, no special room shielding is needed, and it allows for mobility between operating rooms. In a pilot study of 375 patients, some receiving Intrabeam as a boost dose along with external radiation and some receiving Intrabeam alone, there were only two cancer recurrences, each of which could have been avoided with tighter selection criteria in the protocol. There are 10 sites in the U.S. currently using the Intrabeam IORT, and with the company's new "rent-per-case" program more are expected.
Intraop Medical (Santa Clara, California), which just went public a week before the ASBS meeting, also exhibited for the first time at the meeting with its Mobetron, a portable electron accelerator used intraoperatively to deliver electron radiation to the lumpectomy cavity. The room does not need to be shielded, so the Mobetron can be moved from operating room to operating room. In Europe, more than 1,000 patients have been treated using a single dose only and followed for up to 3 years with no local recurrence.
All of these partial breast irradiation techniques deliver radiation to the place where cancer is most likely to recur, thus providing better local control because cancer cells are killed on site. In addition, these techniques minimize the negative cosmetic effect that radiation can cause to the skin and the rest of the breast tissue, as well as allowing a much shorter duration of treatment time.
Genetic testing grows
Genetic testing for risk assessment as well as therapeutic options is a new and growing field that encompasses many different areas of breast cancer. It has been widely accepted in recent years that women who carry the BRCA 1 and 2 genes are at high risk for developing breast cancer, but at this meeting there were two new companies that provide genetic testing that goes beyond BRCA genetic testing.
With a positive BRCA test, the woman has a 45% to 85% lifetime risk of developing breast cancer. In order to better pinpoint breast cancer risk, Intergenetics (Oklahoma City) has studied 11,000 women from six geographic sites and has identified 100 other common genetic variations that may contribute to cancer. Since only about 1% of women develop hereditary breast cancer (BRCA 1 and 2), this additional genetic information, coupled with a brief medical and family history, provides the rest of these women with a numeric and age-specific lifetime breast cancer risk assessment.
The OncoVue swish test uses a simple mouthwash collection system that is sent off to Intergenetics' lab and analyzes the DNA in cheek cells. This allows the woman to be able to know years in advance of a disease diagnosis, and together with her physician, action can be taken to reduce the risk or detect the disease at its earliest stages when long-term survival is the greatest. Intergenetics completed its study last August and is preparing submissions to CLIA in anticipation of being on the market by this fall.
At the other end of the spectrum that is, after a cancerous lesion is removed Genomic Health (Redwood City, California) has developed OncotypeDx a genetic test that helps predict breast cancer recurrence and magnitude of benefit from chemotherapy. Genomic Health, founded in August 2000, is building an oncology-based healthcare services company for physicians and patients to provide individualized genomic analysis of tumor biopsies. It started by addressing breast cancer tumors in women with early stage breast cancer (not pre-cancerous lesions or metastatic lesions) who would most likely be receiving Tamoxifin therapy to reduce the likelihood of recurrence. In evaluating more than 2,600 breast cancer patients, the company found that, unlike conventional wisdom, not all early-stage breast cancers responded to chemotherapy, though most all were receiving it. Its 21-gene test indicates those women who are most likely to experience a recurrence as well as those most likely to respond to chemotherapy findings that are independent of patient age and tumor and correlate far better. Genomic Health is CLIA-approved and CAP-accredited and began national marketing and sales in December 2003.
Minimally invasive biopsies grow
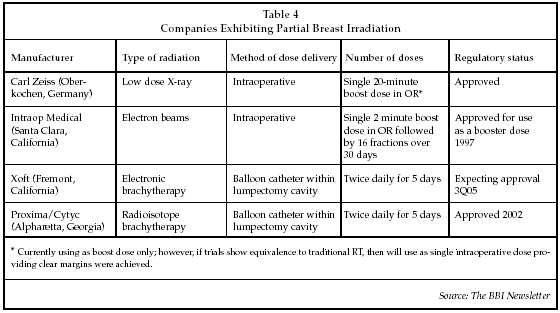
The market trend towards minimally invasive breast biopsies continues to grow, not because open surgical biopsy isn't accurate, but because it is possible to get an equally good specimen without the disfigurement of surgery. Industry estimates that about 45% of all biopsies performed in the U.S. are being performed as an open surgical biopsy, but with companies providing new tools that make the minimally invasive biopsy more accurate and easier to use, it is expected that the trend toward a good cosmetic outcome (minimally invasive biopsy) will continue.
Minimally invasive biopsies can be performed under X-ray stereotactic guidance, ultrasound guidance, or MRI guidance (see Table 5 below) and can use a number of modalities to help capture the targeted specimen such as vacuum, freeze-stick, and radiofrequency cutting. The various options for performing minimally invasive biopsies are becoming so vast that the surgeon can select an appropriate tool based on personal preference as well as case-specific needs.