BBI Contributing Editor
MUNICH, Germany – Congestive heart failure (CHF) is a major and growing worldwide public health problem that is creating demands for a wide range of diagnostic, therapeutic and patient-monitoring technologies in Europe and most other developed regions. According to M. St. John Sutton, of the University of Pennsylvania Medical Center (Philadelphia), who discussed heart failure treatments here during the late-August congress of the European Society of Cardiology (ESC; Sofia Antipolis, France), there are an estimated 22 million individuals with heart failure worldwide, including 5 million in the U.S., 6.5 million in Europe (3 million in the UK, France, Germany, Italy and Spain), and 2.4 million in Japan. More than 330,000 new cases are diagnosed in the five major European countries each year.
The disease accounts for 2% to 3.5% of healthcare costs in the developed regions of the world, and 70% of the cost is attributable to acute heart failure and related hospitalizations. Most heart failure patients are treated with drugs such as beta blockers, ACE inhibitors, angiotension receptor blockers, digoxin and diuretics, but, as discussed by numerous presenters at the congress, a number of new therapeutic approaches have emerged that are having a significant positive impact on patient outcome. The most widely implemented new therapy, cardiac resynchronization therapy, or CRT, is a treatment first introduced about a decade ago that relies on electrical stimulation of the heart, a technology that is playing an increasingly important role in cardiac therapy. More recently, further advances in efficacy have been achieved by combining CRT with implantable cardioverter defibrillator (ICD) backup. Electrical heart stimulation therapies also now extend to applications such as public-use defibrillation for cardiac resuscitation. In the future, cell transplant therapies may provide an additional treatment option that could offer a cure for heart failure rather than relief from symptoms and stabilization of progression.
Another growth area is the treatment of cardiac arrhythmias, particularly atrial fibrillation (AF), using interventional technologies. Such therapies are becoming more popular in Europe, as an expanding array of technologies has made the therapy more effective and widely available. Imaging modalities are playing an important role in enabling improved diagnosis and treatment of many cardiac disorders by allowing physicians to determine the most appropriate treatment strategy and allowing non-invasive monitoring of patient response. Imaging technologies including MRI, cardiac computed tomography (CT), single-photon emission computed tomography (SPECT), positron emission tomography (PET) and cardiac ultrasound are continuing to evolve to meet needs for non-invasive analysis of coronary arteries, assessment of mechanical abnormalities of the heart and assessment of myocardial tissue viability. Imaging modalities, along with a number of new blood tests that measure markers of cardiovascular disease in the blood, are becoming more important in assessing the risk of cardiovascular disease, so that individuals at high risk can be identified and managed to avoid the development of serious conditions.
Arrhythmia, HF treatment market grows
The market for devices used in the treatment of heart failure entered a rapid growth phase recently with the introduction of cardiac resynchronization therapy coupled with ICDs. So far, only a small fraction of the patients who could potentially benefit from CRT, mainly those with severe NYHA Class III or IV disease, have received an implant. In the major countries within Europe, about 450,000 of the 3 million patients with heart failure are candidates for cardiac resynchronization therapy. The cumulative number of patients who have received device implants in clinical trials is less than 5,000 worldwide, according to presenters at the ESC congress. Thus, there is a large potential market remaining to be tapped. In addition to the fact that the results of large-scale clinical studies of CRT have only become available within the last year, the cost of the devices is a major barrier to adoption, especially in Europe. As discussed by G. Boriani, MD, of Ospedale S. Orsola-Malpighi (Bologna, Italy), prices for ICDs have declined from the level of EUR 30,000 that prevailed in 1990 to around EUR 12,500, but nevertheless the total cost for an implant procedure remains around $60,000 based on U.S. data. Prices for CRT-ICDs range from EUR 17,000 to EUR 30,000 according to suppliers, while prices for CRT-only devices are in the EUR 5,000 to EUR 10,000 range. Physicians view CRT therapy as an expensive option and have been very selective so far in prescribing such therapy, particularly CRT combined with ICD implants (which appears to be the preferred modality based on the most recent evidence) for their patients in Europe.
The status of adoption of device-based cardiac rhythm therapy is indicated by recent data on the use of ICD implants in Europe. As shown in Table 1, the number of ICD implants per million inhabitants in Europe, while increasing more than 20-fold between 1990 and 2003, remains well below that in the U.S. at only 60 per million, even though heart failure prevalence is similar in the two regions. As discussed by S.G. Priori of IRCCS Salvatore Maugeri Foundation (Pavia, Italy) at the ESC congress, factors responsible for the lag in utilization in Europe include lack of physician education regarding the benefits of the devices, and reimbursement constraints that have only begun to be relaxed within the past few weeks. In addition, implementation has not been ideal, in that there is a significant difference between the patient population being enrolled in trials of technologies such as CRT-ICD therapy vs. those who are treated with CRT in routine clinical practice.
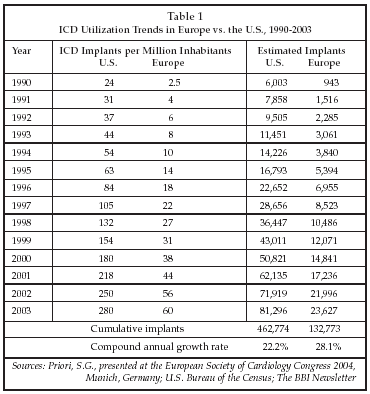
Nevertheless, the indications for ICD use have broadened considerably since the 1980s, when the devices were first introduced, including use of the devices in concert with CRT in heart failure treatment. Trials have demonstrated that ICDs have benefits in both primary and secondary prevention, and that mortality reductions of 36% at one year are achievable in the case of CRT-ICD therapy, based on the results of the recently-completed COMPANION trial. In that trial, total mortality was only reduced in the ICD arm. According to a study described at the ESC Congress by Boriani, the cost per life-year saved of about $26,000 to $27,000 for ICDs is quite favorable vs. other commonly used treatments such as stenting ($26,800) and CABG ($72,900). Furthermore, if device costs continued to decline, the therapy could become even more cost-effective. Some cardiologists have proffered the concept of a $10,000 ICD having only basic features as a device that could help drive increased utilization.
Improving patient selection could potentially provide another significant reduction in overall cost. At present, about one-third of the patients who receive CRT-ICD implants do not respond to the therapy, leading some cardiologists to search for improved methods to qualify patients for treatment, vs. current practices that employ only an analysis of the QRS waveform. Device suppliers, on the other hand, believe that many of the patients who fail to respond do so because the stimulation leads are not optimally placed, and companies such as Guidant (Indianapolis) are developing electronic lead repositioning techniques (via the use of multiple switchable electrodes on each lead) to address that issue. Nevertheless, a major barrier to adoption remains the high up-front cost of ICDs and CRT-ICD devices. Recent changes in reimbursement policy for ICDs in Europe indicate some loosening of restrictions on device use. A new law was just passed in France, for example, that will provide improved reimbursement for ICD implants, and possibly diminish the imbalance in utilization rates between Europe and the U.S., as exemplified by an ICD implant rate of 18 per million in France vs. 180 per million in the U.S. in 2000.
ICD and CRT-ICD devices available in Europe include the recently launched InSync Sentry, the Instrinsic, the InSync II Marquis, and the Maximo from Medtronic (Minneapolis); the Lexos DR-T from Biotronik (Berlin); the Epic HF, Epic + HF and Atlas + HF ICDs from St. Jude Medical (St. Paul, Minnesota), of which about 20,000 have been implanted in Europe; the Contak Renewal 4, Contak TR and Vitality ICDs from Guidant; and the Alto2 MSP biventricular ICD from Ela Medical (Le Plessis Robinson, France), a Sorin Group company.
A new innovation in cardiac rhythm management for heart failure patients, the InSync Sentry, was described at the ESC Congress by Medtronic. The Sentry is an implantable defibrillator that incorporates an added capability, OptiVol, to monitor intra-thoracic impedance, a parameter that is inversely correlated with the degree of fluid build-up in the chest. Increased fluid overload is a highly sensitive indicator of decompensation in heart failure patients and can also be detected by monitoring of body weight. OptiVol is used to measure the flow of electrical current between the pacemaker enclosure and the ICD lead to determine intra-thoracic impedance on a continuous, automatic basis. The technology was evaluated in the Medtronic Impedance Diagnostics in Heart Failure Patients Trial (MID-HeFT), where it was shown to detect deterioration in patient condition 11 days on average prior to the time at which symptoms requiring hospitalization became evident, with a 76% sensitivity and one false alarm in 33 patients. The OptiVol technology can be used with ICDs, CRT-ICDs, or CRT devices, and it is first being commercialized in the InSync Sentry. The Sentry has a built-in audio alarm that sounds for a fixed time each day if a threshold condition is met. In addition, a handheld device is available to obtain a spot readout of thoracic impedance. The programmer used to set the Sentry electrical stimulation parameters also can be used to obtain a stored history of the trend in impedance over time.
The rapid growth of the market for ICDs and CRT-ICD devices is indicated by recent sales trends for Guidant, one of the leading suppliers of ICDs, which reported worldwide sales of implantable defibrillator systems of almost $1.5 billion in 2003, up from $667 million in 2000, representing a compound annual growth rate of more than 30%. Guidant claims a 54% share of the global market for CRT-ICD devices. The total worldwide ICD market, including CRT-ICDs, is in excess of $2 billion, according to suppliers. ICDs with CRT account for about one-third of the ICD implants in the U.S., and are a key driver of recent strong growth in the market. The unit volume market could expand considerably if all patients who are candidates for therapy are treated, although price erosion will likely result in a somewhat smaller dollar volume market opportunity. For heart failure treatment, for example, the number of patients treated each year could more than triple if all of those who have appropriate indications for treatment receive implants.
Another growing treatment modality for heart rhythm disorders in Europe is transcatheter ablation to eradicate atrial fibrillation. As shown in Table 2, the number of catheter ablation procedures for the treatment of atrial fibrillation has grown substantially, almost doubling between 2001 and 2002, according to the results of a worldwide survey presented at the ESC congress by Riccardo Cappato, MD, of Istituto Policlinico San Donato (Milan, Italy). Radio frequency ablation is used in about 90% of all procedures. Worldwide, there are an estimated 8 million patients with atrial fibrillation. Among the companies exhibiting advanced technologies for catheter ablation at the ESC meeting were Endocardial Solutions (St. Paul, Minnesota) and ProRhythm (Setauket, New York). Endocardial Solutions' EnSite System is used to perform diagnosis of rhythm disorders as well as to provide catheter guidance during ablation procedures. The EnSite Array multi-electrode catheter provides non-contact mapping of electrical potentials in as little as a single heartbeat, and allows the operator to display global chamber activation without the need to manipulate a catheter by using a unique balloon catheter that acts as an antenna to collect electrical signals from the heart. Another feature of the EnSite System, NavX, allows CT or MR images to be displayed along with the electrophysiological map to facilitate placement of the ablation catheter. The EnSite workstation is priced at $245,000, and the balloon catheter costs $3,210. For atrial fibrillation guidance, the EnSite Array provides 3-D guidance, visual legend of ablation lesions, and reduction of fluoroscopy time. ProRhythm exhibited its development-stage system using high-frequency ultrasound (HIFU) for atrial ablation. Gas and fluid-filled balloons are used to occlude the veins in the region of the pulmonary vein, and direct contact of the ultrasound probe with the pulmonary veins is avoided. The technology provides uniform ablation, according to the company, in 40 seconds to 60 seconds, and produces less fibrosis post-procedure as compared to RF ablation devices that require catheter contact with the veins. ProRhythm is performing initial clinical studies with the device in the U.S., and plans to begin a pivotal trial in January 2005, with product launch targeted for mid-2006.

New technologies to improve ablation techniques are needed in the market, according to the study of catheter ablation conducted by Cappato. In addition to collecting data on procedure volumes and characteristics, that study also assessed procedural outcomes and complications. The results indicate that outcomes achieved in routine practice are not as positive as those reported in the pioneering clinical studies of catheter ablation for atrial fibrillation. Only 52% of patients became asymptomatic without the use of anti-arrhythmia drugs, and a major complication occurred in 6% of patients. Those rates compare to 85% of patients reported as asymptomatic in the pioneering studies of catheter ablation, and complication rates of 1%. In addition, Cappato said he believes that the survey results underestimate the true complication rates in routine practice. Major complications observed in the study included four early deaths. The high complication rate is particularly troublesome because catheter ablation is typically an elective procedure that is intended to improve a patient's quality of life, and reduce or eliminate dependence on drug therapy, rather than a life-saving procedure for which the risk of complications might be more acceptable.
Another study described at the ESC Congress by Harry Crijns of University Hospital Maastricht (Maastricht, the Netherlands) found that patients in Europe with AF often are subjected to drug therapies that provide little or no benefit, including treatment with anticoagulants to prevent stroke in patients not at risk for thrombogenic stroke, and treatment with anti-arrhythmia drugs to control fibrillation in patients who do not have symptoms of atrial fibrillation. In addition to the excess cost associated with inappropriate treatment, patients are also subjected to needless risks, since anticoagulant therapy carries a significant risk of bleeding, while drugs to suppress atrial fibrillation can cause heart block leading to sudden cardiac death. The researchers attributed the inappropriate use of drug therapy to a failure of clinicians to follow established European practice guidelines.
Advanced technologies for monitoring arrhythmia patients were described at ESC that are helping physicians improve patient management and ensure that appropriate therapy is being provided. For example, Biotronik exhibited its Home Care technology, which provides continuous non-invasive monitoring of patients with ICDs and pacemakers, including heart failure patients, by transmitting data on heart rate, pacing parameters, daily activity and episodes of atrial fibrillation to a monitoring center via the cellular telephone network. The implanted device includes a radio frequency transmitter that sends data to a specialized cell phone, which then transmits the data to a service center in Berlin operated by Biotronik. Physicians can access the data from the service center via the Internet. A two-year service package is included in the price of the implanted device. According to Biotronik, 60% of the ICDs it sells, as well as 10% of its pacemakers, now have the Home Care package, and approximately 2,500 patients are now being monitored worldwide. Medtronic offers a similar monitoring service, CareLink, for its implantable cardiac arrhythmia therapy devices.
CardioDynamics (San Diego) exhibited its Niccomo monitor, which provides the capability to monitor ECG, heart rate, systemic vascular resistance and PEP along with non-invasive cardiac output, expanding the range of available parameters as compared to those available in the company's BioZ monitor. The Niccomo was added to the CardioDynamics product line in April via the acquisition of Medis GmbH (Ilmenau, Germany), the leading supplier of impedance cardiography equipment in Europe, enhancing CardioDynamics' leadership position in the growing non-invasive cardiac output market. Cardiac output is a key parameter used to monitor heart failure patients, and non-invasive monitoring is becoming more widely used to assess patients outside of the critical care setting, as well as in some intensive care settings as an alternative to higher-risk invasive monitoring. Other suppliers of non-invasive cardiac output monitoring systems in Europe include CNSystems Medizintechnik GmbH (Graz, Austria) and Analogic (Peabody, Massachusetts). The EUR 40,000 CNSystems Task Force Monitor tracks cardiac output (via impedance cardiography), blood pressure, peripheral resistance, heart rate, and other parameters, and is often used in the diagnosis of asyncopy. Analogic introduced a new non-invasive cardiac output monitor, the LifeGuard ICG monitor, a highly compact portable system priced at $15,000. Disposable electrodes are priced at $10 per pack. The system is targeted for use in the emergency department, post-operative care, and for titration of drugs used in heart failure treatment. Analogic is seeking distributors for the system in Europe.
While technologies such as CRT-ICDs and improved patient monitoring, along with more effective treatment of acute coronary syndrome patients to minimize the impact of myocardial infarction on the heart are helping to improve outcomes in CHF patients, researchers in Europe are continuing to investigate next-generation approaches with a goal of achieving a cure for the disease. At the ESC Congress, leading researchers provided an update on the status of cell transplantation therapy for heart failure. The field has become embroiled in controversy recently as a number of scientists have questioned the results of studies purported to demonstrate that cells derived from bone marrow or other non-embryonic sources can differentiate into cardiac muscle cells. As discussed by Phillipe Menache, MD, of Hopital Lariboisiere (Paris), there is no evidence of conversion of adult hematopoetic stem cells to cardiomyocytes. Some studies which appeared to show stem cell conversion likely were flawed by the use of analytical methods (immunohistochemical techniques) that were not sufficiently specific. Consequently, according to Menasche, it will be necessary to use cells such as embryonic stem cells or fetal cardiomyocytes to obtain true regeneration of cardiac muscle cells via cell implants.
While recent studies by Menasche have demonstrated that some improvement in cardiac function is achievable using adult hematopoetic stem cells, the improvements have been modest. The use of embryonic stem cells is not without its drawbacks, however. In addition to ethical and political issues related to embryonic stem cell use, it remains difficult to propagate human embryonic stem cells in culture, and less than 10% of the implanted cells survive following transplant. The use of cells other than embryonic cells is thus still being pursued. Some promising results were reported at the ESC Congress by Hans Dohmann, MD, of Pro-Cardiaco Hospital in Brazil, using cells derived from bone marrow. Dohmann has conducted two trials using injected adult autologous bone marrow mononuclear cells including a subgroup of five patients with indications for heart transplant. Some 30 million cells were injected in each patient, using the NOGA catheter from Cordis Endovascular (Miami Lakes, Florida) for guided cell delivery. No arrhythmias were observed at one-year follow-up, and heart function improved sufficiently so that the patients were no longer transplant candidates. Some transplanted cells strongly expressed troponin, a marker that is characteristic of cardiac muscle tissue. The researchers are planning larger clinical trials to confirm their results.
One company is involved in Phase I and II trials with a system for isolation of stem cells for use in cardiac therapy. Miltenyi Biotec (Gladbach, Germany) has developed the CliniMACS System, which can be used to isolate a variety of cell types from bone marrow, and which is being used in some ORs in Germany during coronary artery bypass procedures to isolate cells from the patient which are then implanted in an effort to improve cardiovascular function after the operation. The EUR 25,000 system uses magnetic particles coupled to monoclonal antibodies to isolate specific cell subpopulations, such as CD133-positive stem cells. Cost for consumables used in the procedure is about EUR 2,100, and the process takes two to 2-1/2 hours.
Advances in cardiac imaging in Europe
New developments in non-invasive imaging of cardiovascular disease were described that are likely to have a significant impact on diagnosis and therapy in the future at the ESC congress. Philips Medical Systems (Eindhoven, the Netherlands) exhibited new cardiac magnetic resonance (CMR) technology called Easy Coronaries that provides 3-D visualization of the coronary arteries. The system employs FreeWave with next-generation SENSE technology, a combination of signal processing techniques that provides an eight-fold increase in scan speed. Easy Coronaries is a work-in-progress that will be available later this year.
Philips is developing another new technology, KT BLAST, also scheduled to be available later this year, which provides improved ability to analyze time-varying images, a key capability for cardiac imaging. KT BLAST analyzes 3-D image data in real time to detect changes, storing only the data that varies from one image to the next. As a result, the time required to acquire an image is reduced by 5-fold or 8-fold, depending on the amount of motion in the image. The technology provides a qualitative, high-resolution view of the entire heart using MR imaging.
Philips also introduced Achieve MR at the ESC congress, which provides monitoring of patient vital signs as an integral feature of the magnetic resonance imaging system. A full-function patient monitor with ECG, non-invasive blood pressure, invasive pressure, and pulse oximetry measurement capabilities is built into the Achieve MR system, allowing the patient to be monitored continuously during imaging procedures, an important feature for patients undergoing stress testing, and also improving efficiency in the imaging suite.
MR has a number of features that make it particularly appealing for cardiovascular imaging. Its 3-D capability allows volumetric analysis to be performed, making it possible to assess ejection fraction and valve motion. In addition, the use of gadolinium enhancement, an off-label application in Europe at present, allows assessment of myocardial viability. As a result, MR is now being used to track the effects of cell transplants in heart failure patients. MR also is a particularly attractive method for pediatric cardiology, since it avoids exposure of the patient to ionizing radiation. Other potential applications now under investigation include imaging of blood clots using gadolinium-labeled microparticles targeted at fibrin, as well as imaging of atherosclerotic plaques with targeted particles. Kereos (St. Louis) is one of the leaders in the development of magnetic particles for use in MR imaging, and is partnering with Bristol-Myers Squibb (New York) to develop an MRI agent for the detection of unstable plaque. The Kereos agent uses 100 nanometer to 300 nanometer particles containing gadolinium along with a surface coating that provides preferential targeting. For example, lipid-coated particles can be used to target lipid-rich plaque, and particles coated with agents that bind to fibrin can be used to image clots. As discussed by S. Wickline of Washington University (St. Louis), targeted nanoparticles can be used to image ruptured plaques, blood vessel damage and vessel inflammation due to balloon injury. Human clinical trials of the Kereos agent for imaging of atherosclerotic plaque are planned for next year. An atherolytic agent also is under development by Kereos, which relies on the ability to incorporate drugs into the lipid coating of the nanoparticle. After the particles target plaque, a naturally occurring process results in an exchange of the particle coating with cell membranes in the plaque, resulting in targeted delivery of the drug.
Another factor contributing to the increasing use of MR in cardiovascular imaging is its improving cost-effectiveness. According to data presented by F. Rademakers of Catholic University (Leuven, Belgium), while CMR remains one of the more expensive imaging modalities with a cost-effectiveness index of 5.51, it is much less costly than PET imaging, which has an index of 14.03, and only slightly more expensive than SPECT (3.27) or CT (3.1). Other researchers described using CMR to assess the size of myocardial infarcts and to determine which tissues have been irreversibly damaged in a heart attack. Leading suppliers of CMR imaging equipment in Europe include Philips, GE Healthcare (Waukesa, Wisconsin) and Siemens (Munich, Germany). Siemens exhibited its Magnetom Espree MR imaging system, the first open-bore MR system with CT-like dimensions. The 1.5 Tesla system is particularly useful for assessment of cardiac perfusion, and is preferred for use in interventional procedures even though it does not provide open-MR access to the patient. Although Siemens was the first company to introduce an open MR system, only three were placed in three years because of the product's high cost and issues with installation in available sites that significantly limited the number of potential placements. Siemens now is emphasizing its short-bore systems, such as the Espree, for interventional imaging, which allow the patient to be easily moved in and out of the imaging tube so that MR image guidance can be used in routine cardiovascular surgery procedures.
Advances in cardiac ultrasound imaging were described at the ESC congress by GE Healthcare. A technique known as strain rate imaging is attracting considerable interest as a means of displaying tissue deformation in the heart, and can pick up abnormalities that are not visible on standard echocardiography images. As discussed by G.R. Sutherland of St. George's Hospital (London) at a GE-sponsored symposium at the ESC gathering, strain rate ultrasound imaging may provide a new method for the assessment of ventricular function, as well as for assessment of infarct size and monitoring of reperfusion therapy. The technique can be used to detect ischemic tissue, and may prove useful in the assessment of tissue viability. It also has proven to be easier to implement than tissue Doppler imaging, another ultrasound technique that can be used for tissue characterization which is offered by other suppliers of ultrasound equipment. GE Healthcare also exhibited a new portable ultrasound imaging system, Vivid i, which will be launched late this year or early next, and will provide the features of a full-function ultrasound system in a 5 kg battery-operated unit priced at EUR 60,000 to EUR 80,000.
CardioMag Imaging (Schenectady, New York) exhibited the CMI Magnetocardiograph, a new system for assessing patterns of electrical activity of the heart, with applications in diagnosis of myocardial infarction as well as in assessment of fetal heart abnormalities. The CMI system received 510(k) clearance in the U.S. in July based on demonstration of substantial equivalence to ECG and magnetoencephalography. According to CardioMag, spatial patterns of electrical activity begin to show changes when a pathological condition develops in the heart because the affected areas are bypassed by normal electrical currents. Such changes are not detectable on conventional ECG traces as early as with magnetocardiography. The $595,000 system has been placed in one hospital in Italy and one in China so far, and the company is performing multi-center trials to produce data to justify reimbursement, which it believes will be in the range of EUR 450 to EUR 500 per procedure. The primary application is in the management of acute chest pain patients, to allow earlier and more definitive diagnosis of MI as compared to that available with standard ECG.
