BBI Contributing Editor
Philadelphia, Pennsylvania Tests for cardiovascular disease and the growing use of genomics and molecular testing in the clinical lab were major topics at the annual meeting of the American Association for Clinical Chemistry (AACC; Washington), held here in July. As shown in Table 1, the global market for products used in clinical diagnostics, including products for lab testing as well as point-of-care testing products, totaled $22.9 billion in 2002, with the U.S. accounting for about 43% of the total. While the overall market is growing at only 4% to 5% annually, segments such as molecular diagnostics and cardiac markers have continued to exhibit strong growth, becoming increasingly important segments for suppliers. The applications of molecular testing in clinical diagnostics are beginning to expand to include testing for genetic diseases and cancer, while infectious disease test menus expand beyond chlamydia and gonorrhea to include hepatitis C viral load assays, HIV and HCV genotyping tests, and tests for West Nile virus.

Cardiac testing is growing on a number of fronts. While adoption of troponin tests for the diagnosis of myocardial infarction continues to grow, new markers such as brain natriuretic peptide (BNP) and proBNP are among the fastest-growing new products in the industry. A new group of tests is now emerging that promises to allow earlier detection of heart attacks, potentially detecting an infarction before irreversible tissue damage occurs. Even more importantly, tests such as high-sensitivity CRP may soon help physicians identify those individuals at high risk for a heart attack who do not have abnormal lipid profiles, a group that may comprise up to half of all those who experience a myocardial infarction.
Other important developments described at the AACC conference include advances in cancer diagnosis, involving both immunological methods as well as molecular techniques, and developments in point-of-care testing that are changing the competitive landscape for suppliers in that segment.
Continued growth in molecular testing
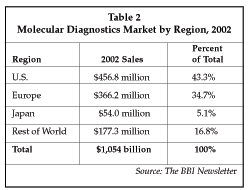
The molecular testing segment of the clinical diagnostics market now exceeds $1 billion worldwide, as shown in Table 2. The market expanded 22.5% in 2002, and growth is forecast to average about 11% over the 2003-2008 period, with sales reaching $2 billion by 2008. Based on a new survey of the U.S. molecular diagnostics market conducted by Enterprise Analysis Corp. (Stamford, Connecticut) and presented at an AACC press conference, test volume increased 14% between 2001 and 2002 in the U.S., and the mix of tests also changed, with 76% of the 51 labs surveyed adding new tests during the past year. On average, clinical molecular diagnostics labs perform 15 different assays, but the number is certain to expand since 80% of the survey labs stated they plan to add more new tests in the coming year, and test volume is expected to grow an additional 14%. The highest volume test is chlamydia, but tests including HIV viral load and human papilloma virus (HPV) are exhibiting rapid growth and are expected to comprise a larger share of total volume in the future. Average test volume per lab is 4,400 per year, and 68% of all labs use the pcr assay technology from Roche Diagnostics (Indianapolis, Indiana), demonstrating the leading market position of that company in molecular diagnostics. One indicator of the expansion of test menus in molecular diagnostics is the survey finding showing that a new genetic test for cystic fibrosis was added by more labs than any other during the past year, although infectious disease tests for HCV viral load and HCV genotype also ranked highly.
Test volume for cystic fibrosis increased an average of 40% from 2001 to 2002 among the surveyed labs, and additional expansion is likely since less than 25% of all labs surveyed currently perform the test. Test volume for cancer-related tests is also exhibiting strong growth, ranging from 5% to 30% among the labs included in the survey. Overall, the utilization of molecular testing is not only increasing, but the scope of testing also is expanding to include a number of higher-priced tests, driving even higher growth in the dollar volume market.
The technologies employed in molecular testing also are evolving. The use of analyte specific reagents (ASRs) is becoming more widespread, and 80% of labs are interested in replacing existing home-brew assays with ASR tests using products from leading suppliers including Roche Diagnostics. Another new development is the use of microarrays in clinical testing, allowing a large number of tests to be performed simultaneously. ASR microarrays are now available from two suppliers, as shown in Table 3 below, and more are in development. At present, the devices are being used to analyze only a small number of gene sequences at a time, up to 25 in the case of the CF microarray from Roche. However, microarray technology can potentially allow many more tests to be performed simultaneously, enabling a fundamentally new approach to diagnostics to be implemented. At present, microarrays have certain drawbacks that limit their utility in clinical testing, including high imprecision (~30%) as well as high cost (e.g., $1,350 for the Nanogen microarray for CF testing). Costs are beginning to drop, however, as unit volume grows, and as the number of suppliers of devices for clinical use expands. The devices may have important applications in cancer diagnosis, where researchers are now using them for discovery of new markers. Furthermore, the ability to analyze very small sample volumes to detect hundreds or even thousands of genetic markers simultaneously is already being used to perform new types of tests such as breast cancer detection and could provide important advantages in the emerging field of pharmacogenomic testing.

New molecular testing products exhibited at the AACC conference for the clinical lab included new sample preparation and purification systems from Abbott Diagnostics (Abbott Park, Illinois), DNA Research Innovations (DRI; Kent, UK), Tecan (Durham, North Carolina), Promega (Madison, Wisconsin), Qiagen (Valencia, California) and Protedyne (Windsor, Connecticut). Abbott exhibited the M1000, a $140,000 system for nucleic acid sample preparation that was launched in the U.S. during the last week of July. The system can process samples for use in a wide variety of tests including cystic fibrosis assays, HIV-1 genotyping tests, HCV viral load assays and a number of cancer-related molecular diagnostic tests. The system can accept blood, sputum, buccal, and other samples, and can process 48 specimens in two hours. It accepts multiple tube types and uses reagent packs to simplify set-up. Processing cost is about $8 per sample.
DRI demonstrated the Charge Switch technology for DNA preparation from blood and buccal cell specimens. Charge Switch is a new patented technology for nucleic acid purification that employs magnetic beads with a switchable positive charge on their surface, allowing specific binding of the negatively charged backbone of DNA in solution. The beads are then captured magnetically and washed, and the DNA is subsequently eluted with a low salt, high pH solution. Total protocol time for blood samples is 10 minutes, and buccal cell samples can be processed in 25 minutes. Tecan previewed the Freedom Evolyzer, a new platform for nucleic acid diagnostic sample preparation that is currently under development. Qiagen has introduced the BioRobot EZ1, a lower-priced DNA purification system for smaller labs priced at $28,000. The EZ1 can process six samples in 15 minutes, but does not process RNA samples.
Protedyne exhibited the BioCube System, a high-capacity, high-speed system for automation of nucleic acid sample preparation, priced in the low $200,000 range to start. The BioCube has primarily been used by pharmaceutical research labs and academic research labs in the past, but the company is now finding strong interest among high-volume clinical labs, particularly large reference labs. The BioCube includes a built-in web interface and accepts a wide range of specimen types including microscope slides, microplate specimens, and tubes. Promega also exhibited the MagnaSil KF Genomic System, a compact $13,000 processor that can perform DNA purification on 15 samples in 25 minutes and is designed for use with the KingFisher mL Magnetic Particle Mover manufactured by Thermo Electron (Waltham, Massachusetts). Nucleic acid sample preparation remains the most difficult aspect of molecular testing for the clinical lab, and there is strong interest in new systems that can help automate that task effectively and at low cost.
New applications for molecular testing that are helping to stimulate the demand for automated sample preparation systems include cystic fibrosis screening tests as well as cancer diagnosis using genomic methods. Cystic fibrosis screening is now an established and rapidly growing application for molecular labs. Cancer diagnosis and guidance of cancer therapy also are beginning to emerge as significant growth areas in molecular diagnostics. Analysis of the BRCA 1/2 mutations in breast cancer and analysis of her-2/neu gene expression in breast cancer are the most widely used molecular cancer tests in clinical labs, but studies of global gene expression profiling to identify new oncogenes and tumor suppressor genes promise to significantly expand the range of applications of molecular testing in oncology. As described in a plenary lecture by Barbara Weber, MD, of the University of Pennsylvania (Philadelphia, Pennsylvania), studies using genomic microarrays have allowed identification of gene expression profiles associated with breast cancer, ovarian cancer and neuroblastoma. Using data from the Human Genome database, the functions of many of the genes affected in cancer can now be identified, allowing changes in gene expression to be related to changes in biochemical profiles. Weber has identified a gene known as HoxA9 that appears to play a role in a majority of breast cancers by perturbing the TGF-beta pathway. In neuroblastoma, the use of comparative genomic hybridization has allowed identification of specific gene deletions that are associated with the probability of progression. Some hospital labs are now beginning to change their approach to cancer classification to use microarrays rather than conventional histopathology, although those labs are so far in the minority. Before microarray profiling can be adopted on a widespread basis, Weber believes that improvements in precision and reproducibility are needed from the current level of 30%-40% with existing devices. Furthermore, truly useful microarrays will be those that allow selection of the most effective treatment for an individual cancer patient and prediction of response.
Infectious disease testing still grows
While cancer diagnostics and genetic testing represent the most rapidly growing segments of the molecular diagnostics market at present, expansion in the range of applications in infectious disease testing, a segment that comprises about 95% of the current market, is far from over. One new application that is being adopted by many labs is testing for hepatitis C virus (HCV), including HCV viral load testing and HCV genotyping. HCV genotyping tests are used in patients with HCV infection to decide how long to treat with agents including pegylated interferon alfa and ribavirin, while viral load tests are used to determine if the infection is resolved. Two FDA-cleared HCV viral load assays are now available, including the COBAS Amplicor HCV Monitor Test v2.0 from Roche Diagnostics and the Versant HCV 3.0 bDNA assay from Bayer Diagnostics (Tarrytown, New York). Roche is developing a new real-time PCR HCV assay that will run on the TaqMan platform, and that could simplify HCV RNA testing by eliminating the need for separate qualitative and quantitative tests. HCV infection has emerged as a major public health problem worldwide, with an estimated 170 million to 200 million carriers, including three to four million in the U.S. Approximately 70% to 85% of HCV-infected individuals will develop chronic hepatitis C infections, and 16% to 20% of those patients will progress to liver cirrhosis after 20 years. HCV infection is now the most common reason for liver transplantation, and projections indicate that the need for such transplants will increase over 500% between 1998 and 2008.
While the high and growing rate of HCV infection is clearly a major public health concern worldwide, there is the potential to reduce the impact of the disease since the standard anti-viral treatments are proving quite effective. Over 50% of patients can be cured with ribavirin/interferon therapy. However, the drugs have significant side effects including fatigue, headache, insomnia and nausea occurring in 22% to 54% of patients, as discussed by Michael Fried, MD, of the University of North Carolina (Chapel Hill, North Carolina), at an AACC symposium. Furthermore, the treatment must be continued for 24 to 48 weeks, with decisions on the length of treatment based largely on the results of molecular testing. As a result, significant growth in demand is anticipated for HCV molecular tests, helping to drive continued growth in the infectious disease testing segment.
New phase for cardiac testing
Cardiac testing is another segment of the in vitro diagnostics market that is undergoing significant changes. The segment includes cardiac marker tests for the diagnosis of myocardial infarction, tests for heart failure diagnosis and prognosis, and tests for assessment of risk for cardiovascular disease. Cardiac troponin tests are now a well established, although somewhat underutilized, tool for diagnosis of heart attacks in the emergency department, and have revolutionized the ability of physicians to accurately triage patients with chest pain. However, as discussed by Robert Jesse, MD, PhD, of the Medical College of Virginia (Richmond, Virginia), at an AACC symposium, there is still a need for new markers to allow earlier detection since troponin markers do not become elevated until five to six hours post-infarct. In addition, about 5% of patients with myocardial infarction have false negative troponin tests, a rate that equates to about 27,000 cases annually in the U.S. Many test panels for heart attack also include myoglobin to serve as an earlier marker, since the time to positivity for that marker is 4.4 to 5.5 hours following the initial infarction. However, that is still too late to allow optimal treatment since tissue necrosis often has already occurred.
Researchers are thus seeking additional markers that can identify earlier events in the progression to myocardial infarction, such as markers of myocardial ischemia and markers of plaque instability. One approach is to develop more sensitive troponin tests that can allow detection of tissue necrosis at an earlier stage. However, the newest marker to be introduced for early detection of heart attack is ischemia modified albumin (IMA), a compound that is formed when albumin comes into contact with ischemic tissue in the heart or another organ. IMA is detected with the Albumin Cobalt Binding (ACB) test developed and marketed by Ischemia Technologies (Denver, Colorado). The ACB test is available on a number of high-throughput automated analyzers including the Hitachi 911 and Modular analyzers from Roche Diagnostics, and the LX20 from Beckman Coulter (Brea, California). The ACB test was cleared by the FDA in February and launched in Europe about a year earlier.
Another new early marker of ischemia, Nourin-1, is being developed by Nour Heart (Gaithersburg, Maryland). Nourin-1 is a chemotactic factor that is released in ischemia and trauma, and in response to exposure to chemical toxins or infectious agents. It is involved in activation of monocytes and neutrophils in response to ischemia and plays an early role in the inflammatory response to tissue injury. Nourin-1 is released into the bloodstream about five minutes after ischemia begins, allowing it to be used as an early marker, although it is not specific to heart tissue. Perhaps most importantly, animal studies indicate that the marker is released before tissue necrosis sets in, i.e., while tissue damage is still reversible, so rapid initiation of therapy when the test is first positive could eliminate the long-term deleterious effects of a heart attack, such as heart muscle loss leading to heart failure. The existing test method for Nourin-1 is a cumbersome, time-consuming cell migration assay, but Nour Heart is developing an immunoassay that will allow the test to be run more quickly and easily.
Another new test that may have applications in early diagnosis of heart attack is the B-Type Natriuretic Peptide (BNP) assay. BNP is available as a point-of-care test from Biosite Diagnostics (San Diego, California), and has met with strong acceptance in the market. Sales of the Biosite Triage BNP test increased more than 1,000% to $38 million between 2001 and 2002, and the test is now available for central laboratory analyzers, a factor that will lead to further growth in volume. The primary indication for BNP testing is heart failure diagnosis, since the marker is released as a result of the stretching of the left ventricle. However, BNP is also released at an early stage during MI because of the stiffening of the heart wall that occurs in response to ischemia. It remains to be demonstrated, however, that BNP can serve as an effective ischemia marker, in part because some heart attack victims have had a prior MI resulting in heart tissue damage, and thus already have an elevated BNP level.
Whole blood choline is another promising new early marker of myocardial infarction, described at the AACC conference by Oliver Danne, MD, of the University Hospital Charite (Berlin, Germany). Whole blood choline appears to have a role in assessment of prognosis following MI, and also shows value when used in combination with troponin assays as well with high sensitivity CRP tests. The marker can complement troponin because it becomes elevated earlier, primarily because of its smaller molecular size, according to Danne. It also detects some patients with MI who are missed by troponin tests. When combined with hs-CRP, whole blood choline almost doubles the ability to predict adverse events accurately.
Yet another test, being studied by Alan Wu, PhD, of the Hartford Hospital (Hartford, Connecticut), evaluates platelet density as a marker of platelet activation. Platelet activation plays a key role in thrombus formation and thus should be detectable in the formative stages of a myocardial infarction, prior to the blockage of blood flow by thrombosis. Previous studies have shown that the formation of monocyte-platelet aggregates correlates with a subsequent myocardial infarction. Wu is using a 2-D platelet analysis function already available on the ADVIA 120 hematology system from Bayer Diagnostics to measure platelet density as a predictor of MI. While platelet activation will probably not prove to be a highly specific marker of MI, Wu envisions using the test in combination with other markers such as troponins, ischemia markers and other thrombosis markers to improve the early diagnosis of MI.
Another trend in cardiac testing is the increasing availability of point-of-care tests to improve turnaround time for existing markers. Biosite and Roche both now offer POC test systems that allow quantitative cardiac marker results to be generated at the bedside. Biosite, for example, just introduced its Cardiac ProfilER panel, a $45 test that provides results for Troponin I, Myoglobin, CK-MB and BNP in 15 minutes using a whole blood sample. New entrants in the POC cardiac marker arena include LifeSign (Somerset, New Jersey) and SYNx Pharma (Toronto, Ontario). LifeSign is marketing the LifeSign MI qualitative test card, providing results for Myoglobin, CK-MB, and Troponin I in 15 minutes. The company expects a quantitative reader for the test to be available in about six months. SYNx Pharma markets the tests, which were developed and are manufactured by Princeton BioMeditech (Monmouth Junction, New Jersey). The tests will compete with qualitative cardiac marker products from Spectral Diagnostics (White Stone, Virginia).
BNP and proBNP tests for heart failure diagnosis, such as the POC BNP test now available from Biosite, represent one of the fastest-growing segments of the diagnostics market. BNP testing in the central lab is also beginning to expand as a result of the availability of new tests. Roche introduced its NT-proBNP assay for the Elecsys analyzer late last year, and Bayer Diagnostics announced FDA clearance of a new BNP assay for the Centaur analyzer in June 2003. Dade Behring (Deerfield, Illinois) acquired a non-exclusive license to the Roche proBNP technology in February of 2003, and Beckman Coulter acquired a non-exclusive license to the Biosite BNP technology in June 2003, with plans to introduce a BNP assay on the Beckman Coulter immunoassay platform in 2004. Biosite is the only supplier of POC BNP tests at present, but SYNx Pharma announced in late July 2003 that it has acquired a non-exclusive license to the Roche NT-proBNP marker for near patient testing, and plans to introduce a combination Troponin I/NT-proBNP test, the NEXUS Dx CHF, subject to FDA clearance. The test will be manufactured by Princeton BioMedTech (Princeton, New Jersey), and will be priced at $22 to $25 per test, which compares favorably to the reimbursement level of $47.50 per test. The NEXUS Dx test provides either a semi-quantitative visual readout or a quantitative result using a companion reader.
There is some debate regarding the relative merits of BNP versus NT-proBNP. NT-proBNP has a longer half-life than BNP, and the basal level increases with age to a greater degree than BNP. In addition, NT-proBNP is elevated in patients with renal failure. However, both markers can be used to significantly improve the management of heart failure patients, and in particular to aid in the differential diagnosis of heart failure vs. conditions such as COPD and pneumonia. The marker has revolutionized the diagnosis of heart failure in the emergency department, where prompt identification of the condition is essential to prevent further decompensation.
New developments in POC testing
A number of additional new developments in point-of-care testing were announced at the AACC conference. The International Technidyne (ITC; Edison, New Jersey) unit of Thoratec (Pleasanton, California) announced the acquisition of the IRMA point-of-care blood gas/electrolyte/chemistry testing system from Diametrics Medical (St. Paul, Minnesota). The agreement was signed in mid-Jul, and is subject to approval by Diametrics' shareholders. The combination will allow ITC to offer the first product providing a full line of coagulation assays along with critical care tests (blood gas/electrolytes) at the bedside. ITC plans to integrate its coagulation analyzers into the IRMA Data Management System (IDMS) by the end of 2003, and will launch new tests for creatinine and lactate on the IRMA system early in 2004.
Bedside blood glucose testing and self-monitoring of blood glucose is by far the largest segment of the POC testing market. Testing in the hospital setting is expected to grow as a result of recent studies of the impact of more frequent glucose testing to achieve improved control of blood glucose levels (tight glycemic control, or TGC) on patient outcome. A landmark study published by Van den Bergh in the New England Journal of Medicine in 2001 showed significant reductions in mortality (from 8% to 5%), reduced length of stay, and a reduction in the number of interventions as a result of TGC in the intensive care unit. In addition, the incidence of septicemia was reduced by 46%, and there was a 50% reduction in blood transfusions, as well as similar reductions in ventilator support. Other studies have shown even more dramatic benefits, such as a ten-fold reduction in mortality in deep sternal wound infection patients, and a two-fold reduction in mortality (from 5% to 2.5%) in post-coronary artery bypass grafting patients. Surprisingly, non-diabetic patients, who comprise between 74% and 87% of admitted patients, derive the greatest benefit from TGC. However, all types of patients appear to benefit, including patients with stress diabetes, a condition that is associated with hospitalization.
Implementation of TGC leads to higher costs for the hospital as a result of a higher volume of bedside glucose tests, but the benefits in reduced length of stay and lower complications more than offset the added cost of testing. The LifeScan (Milpitas, California) unit of Johnson & Johnson (New Brunswick, New Jersey), one of the leading suppliers of bedside blood glucose systems and the leading supplier in the hospital segment, has implemented a company-wide initiative to educate hospitals and medical institutions about the benefits of TGC. Widespread implementation will benefit most suppliers of blood glucose testing systems, although the overall impact will be minor since products used in hospital testing comprise a small percentage of the total market. In addition, adoption of continuous glucose monitoring systems for hospital use may be stimulated, since those systems can greatly reduce the workload on nursing staff associated with frequent glucose testing.
