CDU Contributing Editor
CHICAGO, Illinois With the U.S. market introduction of the Cypher drug-eluting stent from the Cordis (Miami Lakes, Florida) unit of Johnson & Johnson (New Brunswick, New Jersey), interventional cardiology has entered a new era in which patient outcomes for revascularization procedures may, for the first time, become equivalent to surgery for many patients. Definitive studies providing head-to-head comparisons of drug-eluting stents vs. bypass surgery remain to be completed. However, results of studies comparing prior-generation stents with surgery, presented here in early April during the annual meeting of the American College of Cardiology (ACC; Bethesda, Maryland), along with analysis of comparative studies of drug-eluting stents with bare metal stents, suggest that drug-eluting stents could provide the incremental improvement needed to achieve equivalence with surgery, while still providing the benefits of minimally invasive interventional treatment.
The replacement of surgical treatments with less-invasive transcatheter therapies, already a well-established trend in the treatment of heart disease, has not been limited to the area of coronary revascularization. Additional examples of technologies that have been recently introduced or that are about to emerge into the mainstream practice of interventional cardiology include catheter-based approaches to treating congestive heart failure, carotid artery stenting, interventional repair of heart defects, percutaneous heart valve repair and new approaches to the treatment of peripheral vascular disease using next-generation stent technologies.
As discussed by one of the icons in the field of cardiology, Eugene Braunwald, MD, during the ACC opening session, growth in demand for treatments for cardiovascular disease appears inevitable. The trend is due in part to the aging of the population and continued increases in conditions such as diabetes that predispose patients to heart disease. In addition, inadequate use of preventative therapies for cardiovascular disease is a significant contributor to increases in disease prevalence, according to Braunwald, along with the expanded use of new technologies that have improved survival of myocardial infarction patients but created a population at higher risk for developing other cardiovascular maladies. In contrast to predictions made a few years ago by a developing surplus of cardiologists, the field now is hampered by an inadequate work force as demand for services has increased to outstrip supply. As a result, the market for new technologies in interventional cardiology is expected to experience continued strong growth.
New product development activity is continuing to expand, as indicated by one statistic cited by Braunwald that a new cardiovascular clinical trial is launched every other day. In the future, he envisions increased use of technologies such as pharmacogenomics to help enable more rational and effective use of expensive drug treatments; improved approaches to selecting treatment modalities and preventative therapies, again employing genetic testing; and a growing emphasis on diseases such as heart failure, where existing therapies have had little impact on long-term outcomes.
Heart failure therapy - the next frontier
As shown in Table 1, the number of patients with heart failure has increased substantially over the past six years, as demonstrated by the most recent data available from the National Center for Health Statistics (Hyattsville, Maryland). All-listed diagnoses for patients discharged from U.S. hospitals with heart failure were almost 3.3 million in 2001, up 23% from 1995. First-listed diagnoses totaled 1 million. The number of all-listed diagnoses was essentially stable throughout the early 1990s, then began climbing, reaching the highest level on record in 2001, the most recent year for which data is available. According to the American Heart Association (Dallas, Texas), 4.9 million persons in the U.S. now suffer from congestive heart failure. In part, the increase is attributable to the growing number of patients who are surviving a heart attack because of improved therapies such as primary angioplasty, thrombolytic therapy and coronary stents. Heart attack survivors often suffer some degree of damage to myocardial tissue, resulting in heart muscle deterioration, heart remodeling and a progressive drop in the heart's ability to pump blood.

A number of new technologies for the treatment of heart failure are now under development in response to the epidemic rise in prevalence, aimed at slowing and in some cases reversing the course of the disease. As discussed by Igor Palacios, MD, of Boston, Massachusetts, at the ACC sessions, one treatment approach attracting increased attention is revascularization, particularly approaches that employ percutaneous interventional techniques. While the loss of heart muscle was long believed to be an irreversible process, recent research indicates the presence of hibernating myocardium in some patients, raising the possibility that at least some heart function can be restored if blood flow is provided to an affected area. Palacios recommends that all heart failure patients have angiography to determine if any of their heart tissue is salvageable.
In addition, advances in SPECT and PET imaging, including the use of labeled imaging agents, are improving the ability of physicians to detect viable myocardium that can be salvaged in heart failure patients. Recent trials have shown that heart failure patients treated with coronary artery bypass graft surgery have a three-year survival rate of about 90% vs. only 50% for those treated with medical therapy. Surprisingly, even patients with an initially low ejection fraction, who were previously thought to have no prospects for improvement by revascularization, are now showing benefits. Heart failure patients exhibit improved cardiac function either with percutaneous or surgical revascularization, although so far surgery tends to provide better results. Perhaps most importantly, survival for low ejection fraction patients treated with bypass surgery is equivalent to that for patients treated with a heart transplant. Physicians now believe that the use of drug-eluting stents in heart failure patients could allow outcomes equivalent to surgery to be achieved, while minimizing procedural risk, thus expanding the percentage of heart failure patients who can benefit from revascularization.
Biventricular pacing and defibrillation, using devices such as the InSync and Insync ICD from Medtronic (Minneapolis, Minnesota) and the Contak TR and Contak CD from Guidant (Indianapolis, Indiana), is another approach that improves heart function in patients with heart failure. Based on data from Medtronic, about 15% of heart failure patients (or about 750,000 in the U.S.) can benefit from biventricular pacing to resynchronize the heart, and about 2% of patients (or 100,000 in the U.S.) with associated ventricular arrhythmia can derive added benefit from an implantable defibrillator. At the ACC conference, the results of the COMPANION trial using the Guidant device in more than 1,600 patients in the U.S. were reported, showing that cardiac resynchronization therapy not only improves exercise performance and quality of life, but also produces a clinically significant (19%) decrease in mortality and hospitalization. The 43% reduction in all-cause mortality for patients who received implantable defibrillator therapy using the CRT-D device was even more impressive. Cardiac resynchronization therapy already is widely used: more than 40,000 Medtronic InSync devices had been implanted worldwide as of the end of March. The new results are expected to stimulate increased use of resynchronization therapy and, when appropriate, implantable defibrillation therapy for heart failure.
Technologies that help to reduce the damage to the heart resulting from myocardial infarction are also important tools in the effort to improve the management of heart failure. The use of hypothermia to preserve left ventricular function appears promising based on the results of a pilot study conducted with the Reprieve Endovascular Temperature Therapy System developed by Radiant Medical (Redwood City, California). In the COOL MI feasibility trial using the Reprieve system, a 58.5% reduction in mean infarct size was achieved at 30 days for patients who presented within six hours of the onset of a myocardial infarction. Now, a larger study, the COOL MI Pivotal Trial, will evaluate hypothermia therapy in 400 myocardial infarction patients who also receive percutaneous intervention (PCI) vs. a control group receiving PCI only. Two other companies, Innercool Therapies (San Diego, California) and Alsius (Irvine, California), also are developing hypothermia therapy systems. Innercool has initiated the ICE-IT study, which will also involve 400 patients with myocardial infarction, while Alsius is about to begin clinical trials with its CoolGard 3000 heat exchange catheter for hypothermia therapy in myocardial infarction, and is also developing applications of the system in stroke and fever control. Innercool received FDA clearance for its Celsius Control System for neurosurgical applications in January. The Radiant Reprieve system is also on the market for achieving and/or maintaining normothermia in cardiac surgery patients.
Mechanical support devices, including left ventricular assist devices (LVAD) as well as intra-aortic balloon pumps, are also playing an increasingly important role in heart failure patients. Thoratec (Pleasanton, California), a leader in the LVAD market, received FDA approval in November 2002 for use of its HeartMate VE LVAS. Most recently, favorable evaluations for reimbursement for the system were announced by both the Medicare Coverage Advisory Committee and the Blue Cross/Blue Shield Association Technology Evaluation Center.
Suppliers of intra-aortic balloon pumps, including Datascope (Mahwah, New Jersey) and Arrow International (Reading, Pennsylvania), also have expanded the range of applications of their technologies for heart failure therapy. According to Datascope, some heart failure patients have remained on the IABP system for up to one year, and many use the system for three to eight months as a bridge to transplant. Arrow International demonstrated a new technology at ACC, a fiber-optic aortic pressure sensor to enhance the performance of its IABP system. The use of a fiber optic sensor at the tip of the balloon pump catheter allows high-fidelity, real-time tracking of pressure changes in the aorta, and enables adjustment of balloon inflation parameters using a process called Aortic Flow Timing. According to Arrow, Aortic Flow Timing allows automatic matching of balloon inflation to aortic valve closure, so that the balloon begins to inflate immediately after aortic valve closure. Balloon inflation is adjusted within the period of the heartbeat, optimizing pumping efficiency regardless of whether the beat is regular or irregular. The benefit is a reduction in time spent on the IABP for patients requiring temporary support.
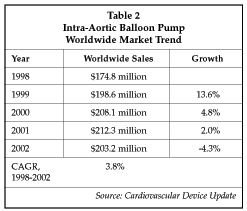
The global market for IABP products has essentially flattened, as shown in Table 2, after exhibiting relatively strong growth in the late 1990s. However, the growing demand for use of the devices in heart failure may help drive growth in the future. In addition, the expected growth in PCI procedures, particularly for patients with more complex, multi-vessel disease, is likely to create some increase in demand. About half (48%) of patients with failed PCI procedures have an IABP placed, and while the failure rate for PCI is now less than 1%, the anticipated expansion in PCI use may result in a greater number of patients who require emergency bypass surgery, and who are thus candidates for IABP placement.
While revascularization, biventricular pacing, LVADs and improved IABPs may help to stabilize at least a percentage of heart failure patients and provide improvement in some, the real need is for a curative therapy that can restore damaged heart tissues and normal heart function. The latest results from studies of cell implantation therapy for heart failure, discussed at the ACC conference, show promise as a potential approach to a cure. As discussed by Cindy Grines, MD, of William Beaumont Hospital (Royal Oak, Michigan) at the ACC sessions, a variety of cell types, including skeletal myoblasts, embryonic stem cells, and bone marrow stem cells, have been used experimentally, and recent results from studies by Francis Pagani, MD, of the University of Michigan's (Ann Arbor, Michigan) cardiac surgery section, have shown that skeletal myoblasts not only survive when transplanted into ischemia-damaged myocardium, but also differentiate into mature myofibers. Other recent reports have shown increases in perfusion and improved heart wall motion following cell transplantation therapy.
However, challenges remain in achieving survival of a sufficient number of implanted cells to restore adequate heart function, and at least one trial, directed by Patrick Serruys, MD, of Erasmus Medical Center (Rotterdam, the Netherlands) using the MyoCell technology developed by Bioheart (Weston, Florida), is on hold because of issues with arrhythmias in patients receiving cell implants in the heart. The trial is expected to resume after the arrhythmia issues have been addressed, probably by using an ICD.
Various technologies are being developed using cell transplants for myocardial regeneration. The Bioheart MyoCell technology, for example, uses autologous myoblasts isolated from a skeletal muscle biopsy and expanded in culture following a proprietary cell selection process. Cells have been implanted both using a percutaneous catheter system (MyoCath) and via surgical techniques. Transvascular (Menlo Park, California) has developed the CrossPoint TransAccess Catheter, a unique delivery device that allows injection of skeletal myoblasts or bone marrow-derived stem cells from the epicardial veins to distances of centimeters into the myocardium. Initial experience with the device in animal studies shows cell uptake by cardiac tissues, new cell generation in the treated area, improvement in electrophysiological characteristics and improved ejection fraction.
Cell transplantation for heart failure treatment has also been evaluated using the NOGA catheter from Cordis to improve the precision of delivery of cell implants. As reported by Emerson Perin, MD, at an ACC press conference, transplantation of autologous bone marrow cells under NOGA guidance in 21 patients with severe coronary artery disease and an average ejection fraction of 20% resulted in significant improvement. Ejection fraction increased to an average of 29%, increased blood flow was observed and angina symptoms improved.
The next advance in cell transplant therapy for heart failure may result from use of improved methods for isolating cells for transplant, and from use of adjunctive drug therapy to stimulate the cell proliferation following implantation. In the Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) study, described by Volker Schachinger, MD, of the University of Frankfurt (Frankfurt, Germany), at the ACC conference, both circulating and bone marrow-derived progenitor cells were delivered to patients via intracoronary infusion following acute myocardial infarction. The cells were selected using processes that resulted in 90% to 100% expressing endothelial cell markers or cell surface markers such as CD34 and CD45. A total of 58 patients were treated, with 29 receiving circulating progenitor cells and 29 receiving bone marrow-derived cells. The goal of the treatment is to prevent the adverse remodeling that often occurs after a heart attack. At four-month follow-up, improved ejection fraction was observed, from 51.6% to 60.1% vs. controls in the first 20 patients treated, and improvement was also observed in regional contractility, end diastolic volume and coronary flow reserve. There were no further deaths in a group of 18 patients followed out to one year.
The use of circulating progenitor cells is perhaps the most attractive approach from the standpoint of avoidance of immune rejection while minimizing the invasiveness of obtaining cells for transplantation. Grines now is involved in a multicenter study that will employ circulating stem cells and will also use granulocyte colony stimulating factor (GCSF), marketed as Neupogen by Amgen (Thousand Oaks, California), to help stimulate progenitor cell proliferation in hopes of enhancing the effect of the therapy.
Another recent development in stem cell therapy for heart failure is the entry into the field by Boston Scientific (Natick, Massachusetts) via an alliance with Osiris Therapeutics (Baltimore, Maryland). Osiris has been developing its mesenchymal stem cell (MSC) technology for multiple applications, including joint repair, bone regeneration and cardiac regeneration. Under the alliance, Osiris will manufacture MSCs and Boston Scientific will sell the cells along with a special injection catheter worldwide. Clinical trials are planned to assess the potential of MSC implants to prevent progression to heart failure following myocardial infarction.
Peripheral vascular therapy grows in importance
Another area of expansion for interventional cardiology is in the treatment of peripheral vascular disease. Although interventional radiologists typically are the primary specialty involved with treating peripheral vascular disease, cardiologists also have begun to perform interventions in the peripheral vessels, particularly in the carotid arteries. Most of the leading suppliers of coronary stents also are developing peripheral stents. For example, Boston Scientific has evaluated the Carotid Wallstent for carotid applications and Cordis is conducting studies with its Precise stent for high-risk groups of patients with carotid stenosis. Other companies developing carotid stents include Guidant, with the AccuLink SE stent and AccuNet embolic protection device; Abbott Vascular (Abbott Park, Illiniois), with the MedNova Xact; and Medtronic Vascular (Santa Rosa, California), with the Exponent carotid self-expanding stent system.
Preliminary results of the ARCHeR trial were presented at the ACC sessions, as well as at the annual meeting of the Society for Interventional Radiology in Salt Lake City, Utah, which preceded the ACC meeting. The ARCHeR trial uses the Guidant AccuLink SE carotid stent as an alternative treatment for high surgical risk patients. As reported by Mark Wholey, MD, at the ACC sessions, the trial has now enrolled 437 patients at 41 sites, all of whom had two or more carotid arteries with a stenosis of greater than 70%, as well as an ejection fraction of less than 30%, making them at high risk for an adverse event if surgery were to be performed. Preliminary 30-day results showed a major adverse event rate of 7.8%, comparable to the rate for low-risk patients undergoing the same procedure. A similar study being conducted in Europe with the Carotid Wallstent has enrolled 61 patients at 14 centers, and results so far are very similar to those from the ARCHeR trial, with a 7.0% major adverse event rate at 30 days.
The results indicate that carotid stenting can be performed safely on high-surgical-risk patients, providing an alternative to carotid endarterectomy and potentially opening up a significant new market opportunity for stent suppliers. There are about 150,000 carotid endarterectomy surgeries performed each year in the U.S. However, another recent study conducted by surgeons at the University of Michigan Medical Center (Ann Arbor, Michigan) found that high-risk patients also could be treated safely with surgery, with an adverse event rate of 4.4% vs. 4.1% for low-risk patients. That study, which involved 429 patients, casts doubt on the theory that one important application for carotid stents is in the treatment of high-surgical-risk patients. Nevertheless, if ongoing trials demonstrate long-term outcomes that are at least equivalent for stenting vs. surgery, many patients may opt for stent treatment in order to avoid a surgical procedure.
Stent suppliers also are focusing on introducing new peripheral stents for biliary, renal and femoral artery applications. The use of stenting to treat those categories of peripheral vascular disease is continuing to increase. Medtronic exhibited its Aurora biliary stent, complementing its Bridge Extra Support renal stent approved by the FDA last December, and Boston Scientific recently launched the Express LD biliary stent, a balloon-expandable device. Boston Scientific claims a 60% share of the self-expanding peripheral stent market, but believes the market is beginning to move toward increased use of balloon-expandable stents.
ev3 (Plymouth, Minnesota) is another important player in the peripheral stent segment, and just introduced the Protege GPS, a self-expanding nitinol stent that features no foreshortening upon deployment. Other stents offered by ev3, which claims to have recently captured the No. 3 market share position in peripheral stents, include the Paramount for renal and biliary applications, and the IntraCoil, the only stent approved for placement in the superficial femoral artery. ev3 acquired its peripheral stent line, developed by IntraTherapeutics, in November 2002 when Centerpulse (Zurich, Switzerland), the former Sulzer Medica, exited the vascular business.
Drug-eluting stents also are expected to have an impact on the peripheral stent market, particularly for applications in the superficial femoral arteries and other small vessels. A sirolimus-eluting stent has been evaluated in the SIROCCO trial with encouraging results, although fractures of the stent struts were noted. A number of self-expanding nitinol stents have been placed experimentally in the SFA, and, based on results of a retrospective study reported at the ACC sessions by Krishna Rocha-Singh of Springfield, Illinois, fractures are a common occurrence. Rocha-Singh evaluated 46 patients who had received implants of 81 different stents, including the Cordis Smart stent, the Medtronic Bridge and the ev3 IntraCoil and found fractures in 50% of all devices except for the IntraCoil. While no clinical events have been traced to the fractures, suppliers are redesigning their stents to eliminate the problem. Cordis has completed enrollment for the SIROCCO II trial, according to Rocha-Singh, and will present results at the Transcather Cardiovascular Therapeutics meeting in Washington in September.
Covered stents, primarily featuring ePTFE membranes along with a stent framework, are another area of development interest within interventional cardiology. Covered stents may have valuable applications in the treatment of diseased saphenous vein grafts, since the membrane can, at least in theory, prevent embolization of much of the debris that is released when deploying bare metal stents. Initial studies in the RECOVERS trial with a covered stent, the Jostent from Jomed (Helsingborg, Sweden), have not, however, demonstrated any benefit vs. bare metal stents. In fact, there was some trend, although not statistically significant, toward worse outcome with the covered stent. A new covered stent, the Symbiot from Boston Scientific, is under development for saphenous vein graft applications in the U.S. market, with introduction targeted sometime in 2004.
Treatment of heart defects expands
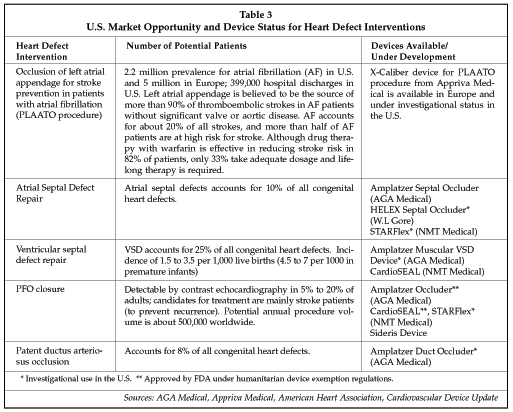
Another important area of expansion for interventional cardiology is the treatment of heart defects such as patent foramen ovale (PFO), atrial septal defects (ASDs), ventricular septal defects (VSDs), and patent ductus arteriosus (PSD), as well as occlusion of the left atrial appendage (the PLAATO procedure) to prevent stroke. potentially there is a large market for devices to treat such defects. For example, treatment of PFO has an estimated market opportunity of 500,000 procedures annually worldwide, as shown in Table 3. Potential procedure volume for the treatment of ASDs, VSDs and PSDs is around 30,000 worldwide, while the PLAATO procedure is potentially applicable to a sizeable portion of the more than 7 million patients in the U.S. and Europe (and more worldwide) with atrial fibrillation. Prices for devices used for the interventional treatment of heart defects range from $2,700 for the Amplatzer Occluder and $3,300 for the Amplatzer Septal Occluder, up to $5,500 for the CardioSEAL device.
Appriva Medical (Sunnyvale, California), now part of ev3, has developed the X-Caliber device for use in occlusion of the Left Atrial Appendage (the PLAATO procedure). The X-Caliber has received the CE mark, and the start of a multicenter, non-randomized trial of the PLAATO procedure in the U.S. was announced at the ACC conference. The trial will assess the effectiveness of the PLAATO procedure in preventing stroke. The PLAATO procedure is potentially applicable to millions of patients worldwide, since more than half of all patients with atrial fibrillation are at high risk for stroke and the procedure addresses the heart defect that is believed to be responsible for a high percentage of strokes in AF patients.
Devices for closure of atrial septal defects, ventricular septal defects and PFOs are now available in the U.S. and most countries worldwide. About 15,000 procedures have been performed with various versions of the devices manufactured by NMT Medical (Boston, Massachusetts), including 10,000 PFO closures. A session at the ACC gathering reviewed clinical experience with the various devices on the market to date, focusing on some of the issues with device placement as well as complications. Overall, complication rates have been very low (less than 0.5%), equivalent to complication rates for surgical treatment of heart defects. Complications have included arrhythmias, cardiac perforation, device migration and hematoma. In addition, about 10% of adults develop migraine headaches after treatment, a complication that is so far unexplained.
The devices have proven effective in preventing strokes, notwithstanding the failure of the FDA to approve NMT Medical's premarket approval application for the STARFlex in September 2002. Ziyad Hijazi, MD, of the University of Chicago Medical Center (Chicago, Illinois), described his experience with treating ventricular septal rupture in myocardial infarction patients using the Amplatzer device from AGA Medical (Golden Valley, Minnesota). Typically, more than 90% of patients who experience such ruptures after a heart attack die in the absence of therapy, and mortality ranges from 19% to 46%, even with surgical closure of the rupture. Previously, Hijazi had experienced promising results using the CardioSEAL device from NMT Medical, achieving a 27% mortality rate in an initial study of 11 patients. More recently, a study involving 17 patients used the Amplatzer device. Importantly, seven of the patients had prior attempted surgical closure that was unsuccessful, leaving them without a viable treatment option. At one-year follow-up, 10 patients are alive and well, with one requiring an additional VSD closure procedure and one dying prior to device delivery, for an overall mortality rate after device placement of 38%. Hijazi concluded that the Amplatzer device is safe and effective for treatment of such defects, but that post-infarct VSD still has a high rate of morbidity and mortality.
Another key competitor in the heart defect treatment device market is W.L. Gore & Associates (Flagstaff, Arizona). Gore is about to complete enrollment in the trial of its Helex septal occluder, and estimates that it will be about a year before the device is on the market in the U.S. The Helex already has been on the market in Europe for about two years, with 500 to 600 devices implanted to date.
