After a long and bumpy path to approval, the U.S. FDA has finally given the green light to Cyclopharm Ltd.'s Technegas combination product a day after the Sept. 29 PDUFA date.
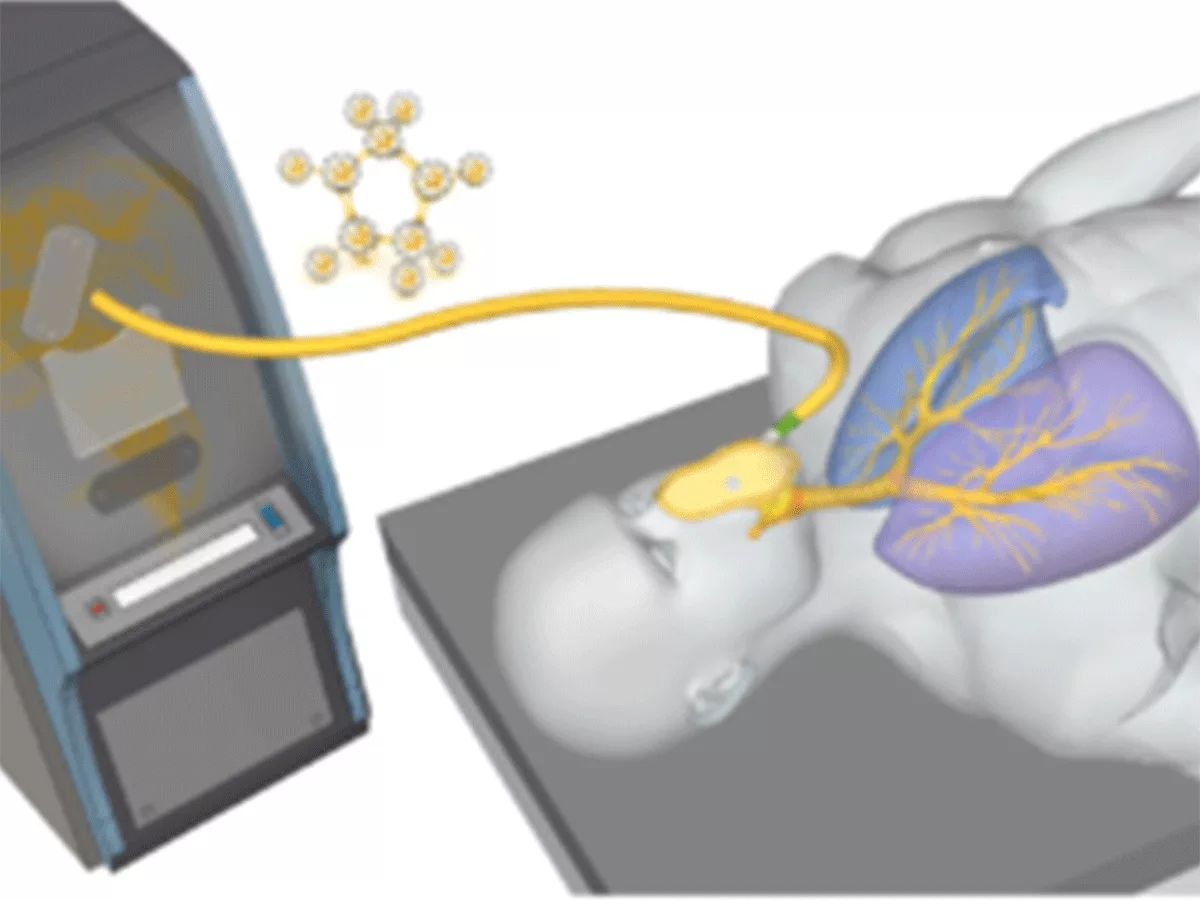
Technegas is a nuclear medicine functional lung ventilation imaging agent that produces a gas-like substance that is inhaled, allowing clinicians to see functional ventilation through the lungs. It uses radio-labeled carbon produced by using dried technetium-99 in a carbon crucible.
The FDA approved the use of Technegas in the diagnosis of pulmonary embolism (PE) as well as for the wider “visualization of pulmonary ventilation.” This broad indication underpins Cyclopharm’s growth strategy for the use of the technology in other respiratory disease states including chronic obstructive pulmonary disease (COPD), asthma, long COVID and lung cancer, Cyclopharm CEO James McBrayer told BioWorld.
After receiving a U.S. FDA complete response letter (CRL) nearly two years ago, the agency finally accepted the NDA in April 2023 and reset the clock. The CRL related to better defining and validating production and delivery of the Technegas particle and other aspects of crucible manufacturing and dosimetry that needed to be addressed before the NDA could be approved. The original user fee date for the NDA was June 26, 2021, and the company had a pre-approval inspection in early April 2021. Some of the items on the CRL related to items identified in that inspection.
Technegas is unique among lung imaging agents, and in the way it is viewed by the FDA, as it is a drug-device combination product, and the Technegas particle, along with the components required for manufacturing, are evaluated together as a drug. The Technegas radioactive particle for inhalation is manufactured at point-of-care within a nuclear medicine department and administered to the patient within 10 minutes.
 James McBrayer, CEO Cyclopharm Ltd.
James McBrayer, CEO Cyclopharm Ltd.
The approval opens for Sydney, Australia-based Cyclopharm the single largest market for Technegas globally, and one which the company estimates to be initially worth about $180 million annually for the diagnosis and management of PE alone.
Pent-up demand in the U.S.
Physicians have been waiting on the approval for so long in the U.S., and there's a “pent up demand,” the CEO said, adding that the U.S. is “behind the rest of the world” in imaging the lung, because Technegas has displaced older technologies, such as Xenon-133 and technetium DTPA, a wet aerosolized nuclear imaging agent.
“Three-dimensional imaging doesn’t work with Xenon, because it's a low energy isotope. And then technetium DTPA is a wet aerosol that starts to pool as soon as it comes out of the nebulizer, and if there's any narrowing of the lungs, it masks a lot of the functional aspects of the lung … so that’s why the U.S. was stuck back in the 1990s.”
“We even had doctors writing to the FDA, and the Society of Nuclear Medicine and Molecular Imaging wrote to the FDA to try to lobby [the agency] to push our approval through,” he said, noting that Cyclopharm received 420 expressions of interest for the product, so it’s not a matter of “getting a sales force together, it’s about the execution in getting the generators to the U.S. and installing them.”
The phase III trial supporting the NDA was a noninferiority structural ventilation study comparing Technegas with Xenon-133 in 240 patients. The trial met both primary and secondary safety and efficacy endpoints.
Technegas is most commonly used in the diagnosis and management of PE. Over the past three decades it has been successfully used in 64 countries worldwide, amassing 4.7 million patient studies. In each of these countries, the technology has become accepted as the preferred nuclear medicine lung ventilation imaging agent.
“By every measure, we're better than the competing products, and that's why we are named in clinical guidelines in Canada, which is our largest country market, and in Europe, which is our largest region.”
Roughly 30% of pulmonary embolisms are fatal if left untreated, and there are about 3 million cases diagnosed per year. Diagnosis is confirmed either through a computed tomography pulmonary angiography or a nuclear medicine ventilation-perfusion study. Nuclear medicine using 3D imaging is the most accurate method of diagnosis.
The commercial rollout will begin immediately, McBrayer said, noting that the company will now complete final assembly of its first wave of 200 generators, with plans for the first air shipments to arrive in the U.S. by early November. Cyclopharm will leverage the company’s sales and operational expertise across 64 countries globally in which the product is already approved and established.
U.S. reimbursement codes are based on established nuclear medicine procedures, which means Technegas can be immediately reimbursed under existing bundled procedural codes for $140 per patient.
We're a little different than some other biotechs in that we do everything ourselves. We're not outsourcing our technology, we're not getting a royalty, we're doing it. We're manufacturing here in Australia, we're going to be rolling it out ourselves with a network of third-party assistants to get us up and running.
Cyclopharm’s stock on the Australian Securities Exchange (ASX:CYC) was up 1.41% on the news, closing at AU$2.87 (US$1.84) at close of trading Oct. 2.