Not only did 2014 bring about a record number of biotech initial public offerings (IPOs), but the stock market performances of many of these graduates was absolutely stellar and enough to vault several into the top-10 positions of "Darlings of Wall Street" for 2014.
In fact, 17 of the top 20 companies going public recorded more than 100 percent plus gains. (See Table 1.)
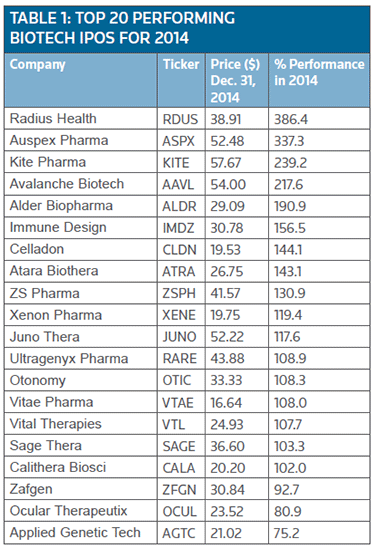
In addition, 60 percent of the 77 U.S. biotech IPOs completed last year finished in positive territory and collectively the group recorded a 37 percent gain – a performance that will no doubt carry over to encourage other companies test the IPO waters.
IF AT FIRST YOU DON'T SUCCEED . . .
Although it took two tries, Waltham, Mass.-based Radius Health Inc. finally made its debut on the public stage in an offering of 6.5 million shares at $8 apiece for gross proceeds of $52 million. (See BioWorld Today, June 9, 2014.)
Unfavorable market conditions led Radius to withdraw a 2012 IPO filing, in which it aimed to raise funds for completing its phase III program testing osteoporosis candidate Abaloparatide-SC, a subcutaneous formulation of a synthetic peptide analogue of parathyroid hormone-related protein that, at that time the company expressed hopes it would stack up well against Eli Lilly and Co.'s blockbuster Forteo (teriparatide).
The company's hopes were realized. Just before the December holiday break, Radius reported that the candidate product had solidly hit its primary endpoint in the phase III trial. (See BioWorld Today, Dec. 23, 2014.)
The news was enough to vault the company's shares (NASDAQ:RDUS) into the lead position among all the biotech IPOs that were completed in 2014 with a whopping 386 percent increase in their value.
Abaloparatide-SC showed a statistically significant 83 percent reduction in the incident of vertebral fractures vs. the placebo group (p <0.0001) in the phase III trial dubbed ACTIVE. It also appeared to show a numerically greater reduction in fracture rate compared to the open-label comparator arm testing Forteo (teriparatide [rDNA origin]), which demonstrated a 78 percent reduction of incident vertebral fractures vs. placebo (p < 0.0001).
Superiority compared to Forteo – currently the only anabolic osteoporosis treatment on the market, with 2013 sales of more than $1.2 billion – isn't a requirement for abaloparatide-SC's approval. But a lower fracture rate, plus better results in bone mineral density (BMD) measures and fewer side effects will be key points for differentiating the product in the marketplace.
About 91 percent of eligible patients from the ACTIVE study have enrolled in an extension study, which will evaluate the reduction in incident vertebral and non-vertebral fractures at up to 24 months as the key endpoints.
POSITIVE RESULTS
Positive late-stage trial results vaulted Auspex Pharmaceuticals Inc.'s shares (NASDAQ:ASPX) 337 percent, enough for second place in the top 20 list. The company is developing a therapy targeting Huntington's disease (HD). Its SD-809 (dutetrabenazine) is an altered form of black-boxed, standard-of-care Xenazine (tetrabenazine, H. Lundbeck A/S). (See BioWorld Today, Dec. 18, 2014.)
The La Jolla, Calif.-based company said top-line data from its phase III trial called First-HD with SD-809 met its primary efficacy endpoint in HD chorea, or uncontrolled movements, and hit secondary goals as well.
SD-809, a small molecule, is a formulation of the vesicular monoamine transporter 2 inhibitor Xenazine that has been modified, with "heavy" hydrogen isotope deuterium. The result is a compound designed to enable less frequent dosing, with improved tolerability, reduced drug interactions and a reduced need for genotyping for drug-metabolizing enzymes. (See BioWorld Today, Nov. 9, 2012.)
The company is continuing development of SD-809 in other movement disorders, with two ongoing pivotal trials in tardive dyskinesia, while pursuing work in Tourette's syndrome, an indication that has not seen an approved drug in more than 30 years.
KITE FLYING
Ranking third in the list is one of the more successful biotech IPOs in terms of funds generated, namely, Santa Monica, Calif.-based Kite Pharma Inc. with $146.6 million. (See BioWorld Today, June 23, 2014, and Aug. 27, 2014.)
The firm's engineered autologous cell therapy genetically engineers T cells to express either CARs, or T-cell receptors. The modified T cells recognize and destroy cancer cells. With the IPO funding, Kite plans to conduct a phase I/II trial next year for lead candidate KTE-C19, a CAR-based therapy, in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
If the outcome is as strong as Kite execs hope, the company would file a biologics license application for accelerated approval of KTE-C19 as third-line therapy in the indication, where it's already designated an orphan drug.
The field is extremely hot with investors. This was evident, not only with the meteoric rise in Kite's share price, but the funds raised for public companies in this space, particularly in December. Kite, for example, upsized a follow-on public offering by 485,000 shares, pricing about 3.4 million shares at $54 each for gross proceeds of about $188.1 million. The company recently announced that it had submitted an investigational new drug application to the FDA to conduct a phase I/II study of KTE-C19, its investigational anti-CD19 CAR T cell therapy, for the treatment of patients with refractory aggressive non-Hodgkin's lymphoma.
Kite's financing came as another CAR T-cell player Juno Therapeutics Inc., of Seattle, priced an eagerly awaited IPO of about 11 million shares of common stock at $24 each, generating $264 million – making it the largest U.S. biotech IPO in the last 15 years. Juno's debut followed one day after fellow cellular immunotherapy firm Bellicum Pharmaceutical Inc. raised $140 million and saw a 25.7 percent hike in share value on its first day of trading.
Juno's welcome was even warmer. The company's shares (NASDAQ:JUNO) closed their first day of trading up 45.8 percent, and by year end they had doubled to close the year up 117 percent.
Juno has clinical work ongoing with CAR T-cell therapies directed against CD19, which has reached the phase I/II stage.
It has its origins in three of the country's top oncology centers – Seattle's Fred Hutchinson Cancer Research Center, New York's Memorial Sloan-Kettering Cancer Center and Seattle Children's Research Institute – and all have been instrumental in helping get the company off on the right foot. In August, it added $134 million in a series B financing round to boost its chimeric antigen receptor, or CAR, and T-cell receptor pipeline. (See BioWorld Today, Aug. 6, 2014.)
According to the firm's S-1A filing, it plans to begin a phase II trial before the end of next year that could support accelerated U.S. regulatory approval in relapsed/refractory B-cell acute lymphoblastic leukemia, a phase I/II trial in relapsed/refractory B-cell non-Hodgkin's lymphoma, and phase I trials for at least five additional product candidates that target different cancer-associated proteins in hematological and solid organ cancers. (See BioWorld Today, Dec. 22, 2014.)
Another hot technology with investors was gene therapy. Levering this excitement was Menlo Park, Calif.-based gene therapy firm Avalanche Biotechnologies Inc., which priced a $102 million upsized IPO, selling 6 million shares at $17. Since its debut it has received an enthusiastic welcome on Nasdaq. Its share (NASDAQ:AAVL) closed the year up 217 percent.
Avalanche is focused on ophthalmic diseases with its lead product, AVA-101, currently under development in a phase IIa trial for wet AMD with top-line results expected this year. The single subretinal injection comprises the AAV2 vector, which contains a gene encoding sFLT-1, a naturally occurring anti-VEGF protein.
Editor's note: For more stories like this, see BioWorld Insight, the weekly news service that provides behind-the-scenes analysis and commentary on the biopharmaceutical marketplace. Subscribe by calling (770) 810-3144 or (800) 477-6307 if calling from the U.S. or Canada or email bioworld at salessupport@thomsonreuters.com.
