Migraine is a complex neurological phenomenon manifesting in painful headaches that can be extremely debilitating which, according to the World Health Organization, ranks it in the top four disabling neurological conditions affecting almost 20 percent of the world's population.
Despite that high prevalence, only a limited number of effective treatment options for migraine exist, which include nonsteroidal anti-inflammatory drugs (NSAIDs) and triptans (5-HTIB/1D agonists). Those medicines, however, are not effective in all migraine patients and they do have side-effect issues. That is why there has been a search for more effective treatments and those afflicted with the condition will have been paying close attention to the just-concluded International Headache Society Congress in Valencia, Spain.
Specifically, interest has heightened around the neuropeptide calcitonin gene-related peptide (CGRP), which appears to play an integral role in the pathophysiology of migraine and was a central topic at this year's meeting.
PROMISING STRATEGY
Inhibiting that small protein, which is involved in the transmission of and heightened sensitivity to pain experienced in migraine, is proving to be a promising therapeutic strategy in preventing the onset of the condition, with several companies involved in the development of antibodies as injectable, prophylactic migraine treatments that target CGRP.
Four companies – Alder Biopharmaceuticals Inc., Amgen Inc., Eli Lilly and Co. and Teva Pharmaceutical Industries Ltd. – are well advanced with their candidate drugs and are vying for a portion of the estimated annual $6 billion and growing market; and all have or will have data readouts from midstage trials this year.
Alder, Amgen and Teva, in fact, presented on Friday at the congress, with Jerusalem-based Teva reporting further data from its chronic migraine phase IIb study evaluating the efficacy and safety vs. placebo of two doses of TEV-48125, an anti-CGRP ligand monoclonal antibody. Teva acquired that antibody, for prevention of both chronic and episodic migraine, through its purchase of Labrys Biologics Inc. for $200 million up front and up to $625 million in pre-launch milestone payments. (See BioWorld Today, June 4, 2014.)
Following a 28-day run-in period, qualifying patients (n=264) were randomized to one of three treatment arms receiving high-dose TEV-48125 (900 mg), low-dose TEV-48125 (675/225 mg) or placebo, given subcutaneously once a month for three months. Subjects had their headache and health information captured daily during the entire study, using an electronic headache diary system. The study was conducted in approximately 60 centers in the U.S.
The study found both assessed doses of the antibody (loading of 675 followed by monthly injections of 225 mg or 900 mg), were significantly superior to placebo in reducing, relative to baseline, the number of hours with headache (primary endpoint – p < 0.05 and p < 0.01). TEV-48125 also significantly decreased the number of headache days of moderate or severe intensity in month three (secondary endpoint – p < 0.05 and p < 0.05).
The study was conducted among 264 highly severe chronic migraine patients who suffered from a mean of approximately 162 headache hours per month (approx. 17 migraine days per month, and around 21 days of headache per month). They had suffered from migraines for mean periods of 18 years. Among the most affected of those patients (upper third), 42 percent reverted to episodic migraine in the 675/225-mg arm and 43 percent in the 900-mg arm, vs. 22 percent in placebo. Overall, by the end of the study nearly 60 percent of patients reverted from chronic to episodic migraine.
"These results with TEV-48125 have not previously been achieved at any phase in chronic migraine. They are highly statistically significant, and provide a solid foundation to advancing the program into phase III," said Michael Hayden, Teva's president of global R&D and chief scientific officer.
SIGNIFICANT RESULTS
Bothell, Wash.-based Alder reported significant results generated from six-month follow-up data on its phase II proof-of-concept trial of ALD403, its anti-CGRP antibody for the prevention of frequent episodic migraine. It was conducted in 163 patients who had on average nine headache days per month. Patients were given single intravenous doses of 1,000 mg of ALD403 or placebo. The primary endpoint was the mean change in migraine headache days from baseline to weeks five through eight, and patients were followed for 24 weeks for additional safety and efficacy analyses.
The results showed that a single intravenous dose of ALD403 demonstrated prolonged efficacy over six months for the preventive treatment of migraine. Over three months, the proportion of patients with a 50 percent, 75 percent and 100 percent reduction in migraine days for ALD403 and placebo was 61 percent vs. 33 percent (p < 0.001); 33 percent vs. 9 percent (p < 0.001); and 16 pecent vs. 0 percent (p < 0.001), respectively.
According Randall Schatzman, the company's president and CEO, the long-term efficacy data are leading the firm to focus on "identifying the optimal once-quarterly dose of ALD403 for both infusion and self-administration formulations . . . we plan to initiate two additional dose-ranging clinical trials in the second half of this year."
Thousand Oaks, Calif.-based Amgen also reported the first results from its global phase II, double-blind, placebo-controlled study evaluating the efficacy and safety of AMG 334, its fully human monoclonal antibody for the prevention of episodic migraine. The study met its primary endpoint of reducing monthly mean migraine days compared with placebo.
In the trial, 483 patients were randomized to subcutaneous monthly placebo or AMG 334 (7 mg, 21 mg or 70 mg) in a 3-to-2-to-2-to-2 ratio, respectively. Patients had a mean baseline of 8.7 migraine days per month. The primary endpoint was the change from baseline in monthly migraine days at week 12. Patients randomized to the 70-mg dose group observed a statistically significant 3.4-day reduction in monthly migraine days, compared with 2.28 days observed in the placebo group (p = 0.021).
IMPORTANT CANDIDATE
In a note to investors, analyst Cory Kasimov of J.P. Morgan said he found the data encouraging at the highest dose (70 mg) and "consider the molecule to be one of the more important pipeline candidates for the company."
Amgen is expected to advance the compound into phase III development later this year.
Data readouts from Lilly phase II trials from its internally discovered CGRP antibody (LY2951742) are expected later this year. The company originally licensed it to Arteaus Therapeutics Inc. for development in a clinical proof-of-concept study. Then, in January last year, based on positive phase II data, Lilly reacquired all development rights.
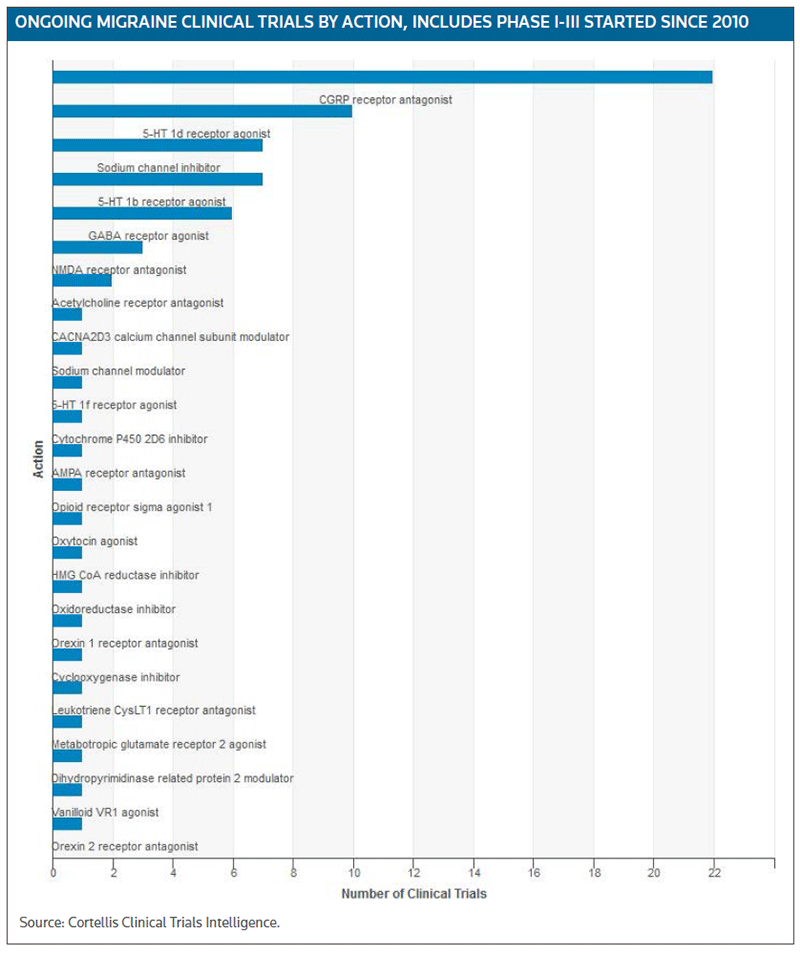
The activity in the CGRP space has certainly sparked investor interest, particularly because each of the compounds being developed by those four companies has blockbuster potential. Migraine has become an important therapeutic category with a a significant number of ongoing trials targeting a diverse range of targets, according to Cortellis Clinical Trials Intelligence. (See Ongoing Migraine Clinical Trials chart, below.)
Burlington, Mass.-based Colucid Pharmaceuticals, Inc., a recent biotech IPO graduate, for example, reported that the first patient has been randomized in its phase III pivotal SAMURAI study for its lead product candidate, lasmiditan, for the acute treatment of migraine headaches in adults. Its objective is to evaluate the safety and efficacy of lasmiditan (100 mg and 200 mg) in comparison to placebo two hours after dosing on freedom from migraine headache pain, the primary endpoint, and on freedom from the most bothersome associated symptoms of migraine (nausea, phonophobia or photophobia), the secondary endpoint. The study is expected to treat up to 1,483 migraine patients with lasmiditan at approximately 70 U.S. sites for approximately 12 months.
Lasmiditan targets 5-HT1F receptors expressed in the trigeminal pathway. Lasmiditan, the company explains, has been given the generic stem name "ditan," which distinguishes it from other drug classes, including triptans, the current standard of care for migraine.
Among other compounds in development, Trevena Inc., of King of Prussia, Pa., reported preclinical data showing that its GPCR-targeted biased ligand candidate, TRV250, could safely activate the delta receptor in the central nervous system (CNS) for potential use in migraine and other CNS disorders. Previous efforts to develop selective delta receptor ligand drugs have been hindered by seizure risk which, in Trevena studies, was associated with delta receptor signals mediated by the scaffold protein beta-arrestin2. Compared to previously described ligands, TRV250 elicits markedly reduced beta-arrestin2 recruitment to the delta receptor. It also was active in models of acute and chronic migraine as well as models of depression, anxiety and pain, including pain associated with central sensitization. The drug displayed very broad safety margins in multiple preclinical species, and is not expected to have addiction or abuse liability associated with mu-opioid agonists like morphine and oxycodone. (See BioWorld Today, May 15, 2015.)