At the end of the third quarter, the industry hit an important milestone with public and private biotech companies collectively raising $25 billion for the nine-month period. The total ranked as the second highest amount of financing dollars raised during the past 20 years. The question at that time was whether the amount of $36.9 billion, generated in 2000, could be surpassed. (See BioWorld Insight, Oct. 6, 2014.)
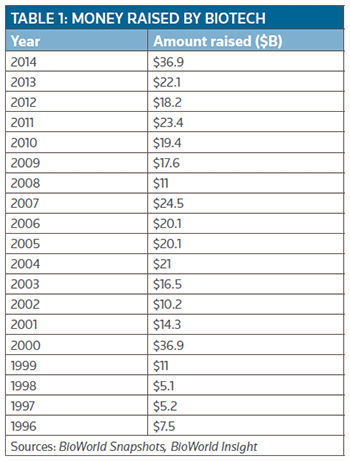 It turned out to be extremely close, thanks to a great fourth quarter with $11.9 billion raised by the time the books were closed on 2014. As a result, the year goes into the history books tied with 2000. (See Table 1, right.)
It turned out to be extremely close, thanks to a great fourth quarter with $11.9 billion raised by the time the books were closed on 2014. As a result, the year goes into the history books tied with 2000. (See Table 1, right.)
The fourth quarter total represented a 79 percent increase on the $6.6 billion that was raised in the third quarter and 93 percent increase on the total generated in the fourth quarter of 2013.
Overall, the $36.9 billion generated in the year was a whopping 67 percent increase over the $22.1 billion that was garnered in 2013. Approximately 62 percent of the total cash generated in 2014 came as a result of public offerings.
PUBLIC OFFERINGS
The 30 public offering transactions in the fourth quarter generated approximately $6.4 billion, which was six times the amount raised through public offerings in the third quarter of 2014 from 17 deals.
For the year, public offerings generated $23 billion, almost twice the $12 billion raised in 2013 and an amount that represents the highest ever raised in the history of the industry. Table 2 (below) shows just how the public offerings total in 2014 has dwarfed those for the past decade. In 2000, the amount raised from public offerings was $14.7 billion.
Significantly boosting the deal total in Q4 was Gilead Sciences Inc.'s pricing of senior unsecured notes in an aggregate principal amount of $4 billion in a registered public offering. The financing was made in three tranches: $500 million of 2.35 percent senior notes maturing in February 2020; $1.75 billion of 3.5 percent senior notes maturing in February 2025; and $1.75 billion of 4.5 percent senior notes maturing in February 2045. The Foster City, Calif.-based company said it intends to use the net proceeds for general corporate purposes, which may include the repayment of certain indebtedness, debt-related payments, working capital and the repurchase of outstanding common stock related to the firm's authorized share repurchase program. (See BioWorld Today, Nov. 14, 2014.)
Taking advantage of a soaring share value Bluebird Bio Inc. (NASDAQ:BLUE), just over five months after netting $95.6 million in its first follow-on offering, raised $259 million from the sale of about 3 million shares priced at $85 each. The gene therapy company had just generated considerable excitement in the space by reporting that four young adults with beta-thalassemia major, a severe blood disorder that leads to life-threatening anemia, were able to produce sufficient hemoglobin to reduce or eliminate the need for chronic blood transfusions after being treated with its gene therapy, Lentiglobin BB305.
 The news sent its shares soaring, enough to see the company's shares grow in value 337 percent during 2014.
The news sent its shares soaring, enough to see the company's shares grow in value 337 percent during 2014.
Bluebird intends to use net proceeds from the recent financing primarily to advance studies in childhood cerebral adrenoleukodystrophy, as well as beta-thalassemia major and sickle cell disease. (See BioWorld Today, Dec. 10, 2014.)
FOLLOWING ON FROM GOOD DATA
Another company that took advantage of a higher share value on the heels of promising clinical data results was San Diego-based Receptos Inc. The company generated $414 million from its public offering of an aggregate of 4.1 million shares of its common stock at $100 each, which included 540,000 shares sold in the full exercise of the underwriters' option.
Receptos is using the net proceeds to fund continued development of its product candidate RPC1063 in ongoing and planned trials for relapsing multiple sclerosis (MS), ulcerative colitis and Crohn's disease, as well as continued development of its in-licensed product candidate RPC4046 in an ongoing clinical trial for eosinophilic esophagitis.
In the fourth quarter the company released top-line data showing that oral sphingosine 1-phosphate 1 receptor (S1P1R) small-molecule immunomodulator RPC1063 met its primary and secondary endpoints in ulcerative colitis in the phase II TOUCHSTONE trial. A month earlier, shares spiked after an abstract of the complete dataset from the phase II portion of the phase II/III RADIANCE trial in relapsing MS patients prompted analyst murmurings of a potential best-in-class S1P1R scenario for RPC1063. (See BioWorld Today, Sept. 15, 2014, and Oct. 29, 2014.)
Receptos watchers soon will be looking for detailed induction and maintenance data from the TOUCHSTONE study, expected in the first half and second half of this year, respectively. The company has further plans for RPC1063 with a phase II study set to start this year in Crohn's disease.
KITE FLYING HIGH
Santa Monica, Calif.-based Kite Pharma Inc. also had a great year, capping it off with an upsized follow-on public offering in the fourth quarter, pricing about 4 million shares at $54 each for gross proceeds of about $216 million. The company is gearing up for a phase III trial with its anti-CD19 chimeric antigen receptor (CAR) T-cell therapy. Kite went public in June with a $146.6 million IPO. (See BioWorld Today, June 23, 2014, and Aug. 27, 2014.)
It also kept up the momentum and in the early days of the new year announced that Amgen Inc. had signed an early stage deal to apply Kite's CAR technology against a set of cancer targets amassed over the years at the Thousand Oaks, Calif.-based biotech. In addition it has entered an exclusive, worldwide license with the NIH for intellectual property related to T-cell receptor (TCR)-based product candidates directed against human papillomavirus (HPV)-16 E6 and E7 oncoproteins for the treatment of cancers associated with HPV infection. The National Cancer Institute (NCI) recently initiated a phase I/II trial of one of those TCR product candidates, targeting the HPV-16 E6 oncoprotein, under a Cooperative Research and Development Agreement between Kite and the NCI. Kite will provide certain clinical, regulatory and sales milestone payments and royalties on net sales of products covered by the license.
PRIVATE PLACEMENTS
The fourth quarter was also an active one for public companies conducting private placements such as registered direct offerings, rights offerings and at-the-market transactions with 68 deals being completed for a collective total of $2.2 billion raised. That was 20 percent shy of the total raised in the third quarter of 2014 but 30 percent higher than the $1.7 billion raised in the same period of 2013. For the year, private placements raised $8.1 billion, 40 percent greater than the $5.8 billion in 2013.
One of the largest private offerings in the period was the $200 million aggregate principal amount of 7.50 percent convertible senior notes due in 2019 conducted by Synergy Pharmaceuticals Inc., of New York. The notes will be convertible into shares of the company at an initial conversion rate of 321.5434 shares per $1,000 principal amount of notes, which is equivalent to an initial conversion price of approximately $3.11 per share.
