Several big pharma companies still have to face the pain of significant revenue erosion over the next couple of years as their blockbuster products lose patent protection and endure the prospects of generic competition. However, by and large most have managed to navigate themselves beyond this tough terrain thanks to creative business strategies designed to secure consistent future growth.
Novartis AG, for example, sees its prescription cancer treatment Gleevec (imatinib) go off patent next year, which will potentially create competition for the current annual $4.7 billion in revenue it generates. In addition, in July the company saw the entry of generic competition for its hypertension drug, Diovan (valsartan) monotherapy in the U.S.
Despite this, in its recently released second quarter financial results, it reconfirmed its outlook for full year 2014 with group net sales expected to grow at a low to mid-single digit rate. Also in order to bolster its oncology franchise the company, following a significant examination of its corporate strategies, announced in April that it would exchange assets with Glaxosmithkline plc (GSK) in a three-way, "super complex" deal. (See BioWorld Today, April 23, 2014.)
Novartis said it was paying $16 billion for GSK's marketed oncology portfolio, while GSK acquires Novartis' vaccines arm for $5.25 billion, and the two also planned to pool their consumer health care businesses in a joint venture. In a separate transaction, Novartis divested its animal health business to Eli Lilly and Co. for $5.4 billion.
TRANSFORMATIONAL
The CEOs of both GSK and Novartis said the deal will be "transformational" for their companies. GSK becomes the world leader in vaccines, while crystallizing the value of its oncology portfolio and becoming the majority 63.5 percent owner of a consumer health care business with combined revenues of £6.5 billion (US$10.9 billion).
Novartis, meanwhile, builds on its position as number two worldwide in oncology. It will have first rights to commercialize cancer products coming through GSK's R&D labs, and realizes value from its vaccines and animal health arms, while being able to focus on its big three businesses in pharmaceuticals, eye care and generics.
Paris-based Sanofi SA also had its share of patent cliff hurdles to overcome, but execs there now believe these issues are behind them. Commenting on its financial results in Q3 2013, CEO Christopher Viehbacher said, "The third quarter marks an inflection point for Sanofi as the impact of the patent cliff ended in August."
Fast forward to its Q2 2014 financial results: Viehbacher noted that its performance in the period reflected consistent execution of its growth strategy and "allows us to slightly adjust upwards our 2014 financial guidance. Based on the solid momentum in our late stage pipeline, we are actively preparing for a wave of new product launches that will further redefine Sanofi as a biopharmaceutical leader."
At the end of Jul 2014, Sanofi's pipeline consisted of 46 new molecular entities and vaccines in clinical development, of which 12 were either undergoing phase III studies or were under regulatory review.
SECTOR-WIDE SUCCESSES
Sanofi's successes have been reflected across the pharma sector and we have witnessed a very active period.
Back in October 2014, Merck & Co. Inc. chairman and CEO Kenneth C. Frazier announced a major strategic restructuring to create "greater efficiencies" for the company. The goal: to put its R&D operations in order and pare its annual operating expenses by approximately $2.5 billion by the end of 2015. The multiyear initiative would identify opportunities that offer the greatest potential return on investment and improve the company's performance. (See BioWorld Today, Oct. 2, 2013.)
Although still a "work in progress," Merck is well on its way to achieving its goals.
In its second quarter financial results, Frazier said, "We delivered a strong first half of the year, making progress in transforming our operating model, fueling innovation and managing costs, while focusing on our best opportunities. I'm excited as we are preparing for a series of important and promising product launches later this year that we believe will make a meaningful difference to patients, healthcare providers and payers, while creating value for society and shareholders."
Certainly it has been a busy quarter for the company. In May it completed filing of its FDA biologics license application for MK-3475 (pembrolizumab), its high-profile programmed cell death 1 (PD-1) inhibitor. The company is seeking approval to treat melanoma patients unsuccessfully treated with Yervoy (ipilimumab, Bristol-Myers Squibb Co.), an indication for which the FDA has granted it both breakthrough designation and priority review status. (See BioWorld Today, May 7, 2014.)
The FDA assigned Merck's application an Oct. 28 PDUFA date. If approved, the experimental therapy has the potential to be the first anti-PD-1 antibody in a new class of immune checkpoint modulators, giving it an initial lead on nivolumab, a competing therapy in development by Bristol-Myers Squibb.
The company also bolstered its hepatitis C virus (HCV) position with the acquisition of Idenix Pharmaceuticals Inc. for $24.50 in cash per share. (See BioWorld Today, June 10, 2014.)
The transaction brings aboard Idenix's IDX21437, a uridine nucleotide (nuc) analog HCV NS5B polymerase inhibitor for which the firm recently reported data from a phase I/II trial, which match up well against Sovaldi (sofosbuvir), the nucleotide analog HCV NS5B polymerase inhibitor from Gilead Sciences Inc., of Foster City, Calif.
With its eyes squarely focused on research, the company off-loaded its Merck Consumer Care, one of the world's largest suppliers of over-the-counter products, to Bayer AG for $14.2 billion in cash.
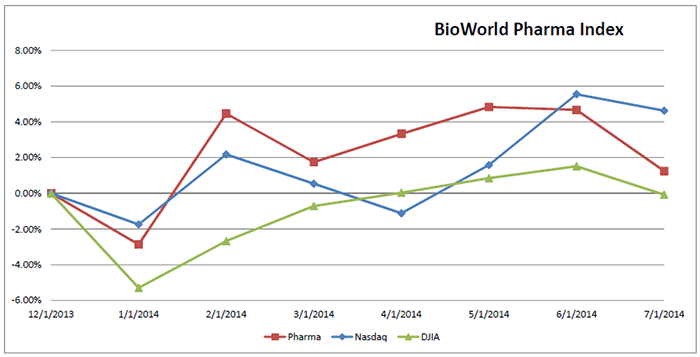
The Street has reacted favorably to these initiatives. The company's share value has risen 25 percent from the time it first announced its major restructuring. It is the leading gainer in the BioWorld Pharma Index, which has grown modestly by 1.25 percent year-to-date. (See BioWorld Pharma Index, below.)
TAX INVERSION PLAY
Pfizer Inc., on the other hand, has seen its share value slip by almost 6 percent in the same period. The company recently failed in its $100+ billion bid to acquire Astrazeneca plc, in a tax inversion play. Most analysts believe that the firm is anxious to make a big deal and CEO Ian Read said on an analyst call to discuss the company's second quarter 2014 results that the company will "evaluate all opportunities, regardless of size."
It did act on one opportunity in June, announcing a strategic collaboration in cancer based on Cellectis' allogeneic chimeric antigen receptor T-cell (CAR T-cell) platform, which will involve an initial modest outlay of about $112 million, as well as research funding, milestones that could reach as much as $2.775 billion total, and tiered royalties on any products that reach the market. (See BioWorld Today, June 19, 2014.)
Pfizer is paying $80 million up front and will acquire a 10 percent stake in Paris-based Cellectis, at a cost of $32.4 million. It also will pay up to $185 million in development, regulatory and commercial milestones per product. The deal gives Pfizer exclusive rights on CAR T-cell therapies directed at up to 15 different oncology targets. Also included under the banner of the deal are another 12 targets selected by Cellectis. Pfizer will have first right of refusal on four of them, and it will provide research funding for these programs as well as for the Pfizer-designated programs. The two companies will collaborate on preclinical development of these therapies.
As pharma companies strengthen their core capabilities, expect to see them continue to be active participants in partnerships and acquisitions. Biotech companies will certainly benefit.
