Big things have always been expected to result from nanotechnology research and now that confidence is starting to pay dividends with potential second-generation therapeutic applications not too far away from becoming available to treat a number of major diseases. Scientific research has advanced our understanding of the many different functions nanoparticles play in optimizing drug delivery. Drawing from experiences of first-generation products, nanomedicine is rapidly evolving thanks to the more effective delivery of encapsulated proven therapeutics and innovative nanoparticles, such as gold and carbon nanotubes.
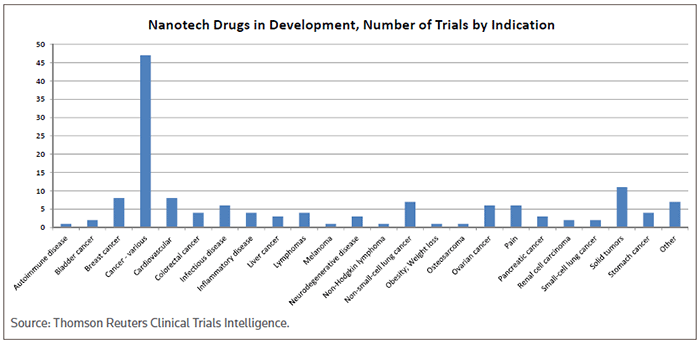
According to a new report from Transparency Market Research, the compounded annual growth rate of the nanomedicine market will grow at a healthy clip of more than 12 percent per annum over the next five years. Key indications served by innovative new therapies shaped by nanotechnology will be in the oncology, cardiovascular and anti-infective fields. (See Nanotech drugs in development, Number of trials by indication, below.)
CLINICAL DEVELOPMENTS
There have been a significant number of clinical developments in the first half of the year that give credence to those estimates. In May, for example, Cambridge, Mass.-based Merrimack Pharmaceuticals Inc. unveiled strongly positive data from its NAPOLI-1 phase III trial with MM-398 in metastatic pancreatic cancer patients previously treated with gemcitabine. The drug is a nanoliposomal encapsulation of the chemotherapy agent irinotecan. Top-line results showed that the combination of MM-398 with 5-FU and leucovorin achieved an overall survival of 6.1 months vs. the 4.2-month survival demonstrated by the control arm of 5-FU and leucovorin alone. The company said it expects to submit a new drug application to the FDA for the MM-398 combination regimen this year.
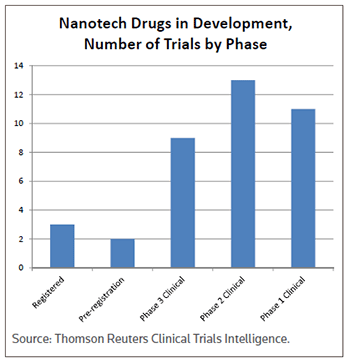 While many of the nanomedicines under development are still in the early phases, a number of compounds have been advanced into mid- or late-stage clinical trials. (See Nanotech drugs in development, Number of trials by phase, left.)
While many of the nanomedicines under development are still in the early phases, a number of compounds have been advanced into mid- or late-stage clinical trials. (See Nanotech drugs in development, Number of trials by phase, left.)
Among those in late-stage trials include NC-6004 nanoplatin from Nanocarrier Co. Ltd., of Chiba, Japan, that is in phase III studies in pancreatic cancer being conducted with partner Orient Europharma Co. Ltd., of Taiwan. The compound is also in phase I trials in non-small-cell lung cancer.
The company also entered a worldwide, exclusive license agreement with Eisai Co. Ltd., of Tokyo, for the rights to develop and market an active compound called E7974 for new drug development. That compound will employ a newly developed technology, called antibody drug-conjugated micelles (ADCMs), which has shown in an animal model study that ADCMs encapsulating E7974 can expand the therapeutic window significnatly, by approximately 10 times.
Cambridge, Mass.-based Cerulean Pharma Inc. is leveraging its dynamic tumor-targeting platform to create nanomedicines that concentrate their payloads inside tumors where they slowly release effective cancer therapeutics from within the tumor cells.
Cerulean's CRLX101 compound employs camptothecin as its payload. The company said it inhibits topoisomerase 1, which is involved in cellular replication, and hypoxia-inducible factor-1a (HIF-1a), which research suggests is a master regulator of cancer cell survival mechanisms thought to promote drug and radiation resistance.
CRLX101 has shown activity in late-stage disease in three different tumor types, relapsed renal cell carcinoma, relapsed ovarian cancer and neoadjuvant rectal cancer – both as monotherapy and in combination with Avastin (bevacizumab, Roche AG).
At the recent American Society of Clinical Oncology meeting in Chicago final data from a phase II trial evaluating CRLX101 as a monotherapy in patients with relapsed ovarian cancer were presented. The trial met its endpoint, with six patients achieving progression-free survival of more than six months. The trial was completed at hospitals affiliated with Harvard Medical School, and the company said it served as the foundation for a trial currently being conducted at the same hospitals in which CRLX101 is being evaluated in combination with Avastin in platinum-resistant ovarian cancer patients.
The company also has an ongoing phase Ib/II trial of CRLX101 in combination with Xeloda (capecitabine, Teva Pharmaceutical Industries Ltd.) and radiotherapy in the neoadjuvant setting for patients with locally advanced rectal cancer.
INDUSTRY INTEREST
Bind Therapeutics Inc., of Cambridge, Mass., a clinical-stage nanomedicine platform company, has received a great deal of industry attention for its Accurins – polymeric nanoparticles that incorporate a therapeutic payload and are designed to have prolonged circulation within the bloodstream. The targeting ligands on the surface of the Accurin bind to specific cell-surface markers. A "stealth" layer using polyethylene glycol protects the Accurin from the immune system.
Although it recently agreed with Thousand Oaks, Calif.-based Amgen Inc. to kill a partnership intended to advance a nanomedicine for treating solid cancer tumors based on the Accurin platform and an undisclosed Amgen compound, with the results of the collaboration "not sufficiently compelling to proceed," the company still has plenty of other "shots on goal."
Bind has collaborations with Pfizer Inc. and Astrazeneca plc, and, just last month, it entered a research agreement with Roche AG to discover nanomedicines using Accurins for the treatment of diseases in therapeutic areas outside of oncology.
Also, commenting on its first quarter financial results, Bind's president and CEO, Scott Minick said, "In a planned interim analysis of the non-small-cell lung cancer (NSCLC) phase II trial for BIND-014, we observed promising preliminary clinical results in six patients with KRAS mutations and intend to add an additional clinical study to further investigate this promising clinical result. Additionally, we completed enrollment in the BIND-014 phase II NSCLC trial on the once every three week schedule and initiated enrollment in the weekly schedule."
The company said it plans to further evaluate BIND-014's activity in additional patient populations, including NSCLC patients with KRAS mutations, and additional tumor types in which there is strong biologic rationale for BIND-014 treatment including cervical, bladder and neuroendocrine cancer.
OTHER INDICATIONS EXPLORED
Although nanomedicines targeting cancer predominate, other indications are being explored. Kala Pharmaceuticals Inc., for example, is focused on the development of innovative ophthalmic products based on the company's mucus penetrating particle (MPP) platform. In June, it announced that it started two clinical trials with its nanotechnology-based loteprednol etabonate MPP (LE-MPP) program, KPI-121.
Kala's MPP nanotechnology platform allows therapeutic agents to pass through the mucus layer of the tear film, facilitating penetration into deeper tissues of the eye, including the cornea, aqueous humor and retina.
A phase III trial (KPI-121-C-001) will evaluate the safety and efficacy of LE-MPP in managing inflammation and pain associated with cataract surgery. Kala aims to enroll approximately 375 patients in 25 centers in the U.S. The trial will dose 1 percent LE-MPP two times daily and 0.25 percent LE-MPP four times daily as compared to placebo in subjects who have undergone cataract surgery and who require treatment of postoperative anterior ocular inflammation.
In addition, a phase II trial (KPI-121-C-002) with KPI-121 will investigate the safety and efficacy of low-dose LE-MPP in patients with dry eye disease.
The company said it believes that nanotechnology "has the potential to transform the treatment of eye diseases in much the same way it has already transformed other therapeutic areas."
Meanwhile, Aadi LLC, a small new biotech company led by Abraxane (nab-paclitaxel) co-inventor Neil Desai, is in-licensing a once-shelved nanoparticle therapy from Celgene Corp. to develop it for oncology and cardiovascular indications, where it plans to initiate clinical studies with its ABI-009 in the treatment of peripheral artery disease and pulmonary arterial hypertension. (See BioWorld Today, May 21, 2014.)
INTO THE PUBLIC ARENA
A clear indication that nanotechnology is on the radar screen of investors is their willingness to back companies working in the space. Several companies, for example, have taken advantage of the wide open initial public offering (IPO) window to step into the public arena.
Bind completed its IPO least year, resulting in gross proceeds of approximately $76 million, which included the exercise of the underwriters' option to purchase additional shares. This year Cerulean braved the uncertain financial climate in April and priced 8.5 million shares – up from the 5 million it originally planned to sell – at $7 each, below its proposed $11 to $13 range. (See BioWorld Today, April 11, 2014.)
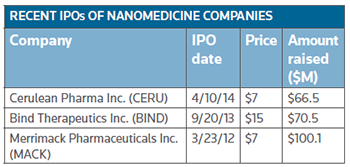 Merrimack priced its IPO of 14.3 million shares of common stock at $7 per share, to raise over $100 million. (See table, left.)
Merrimack priced its IPO of 14.3 million shares of common stock at $7 per share, to raise over $100 million. (See table, left.)
In May, Boston-based Nanoantibiotics Inc., a development-stage company focused on the discovery, development and commercialization of new anti-infective drugs, began trading its stock on the OTC Markets. To combat multidrug-resistant bacteria, the company said it is developing a nano-efflux pump inhibitor and other nano-antibiotics.
Nanomedicine companies also are attracting venture capital. Kala completed a $22.5 million series B financing to fund four trials of the company's mucous-penetrating nanoparticle platform. (See BioWorld Today, April 24, 2014.)
Abingdon, UK-based Midatech Ltd. received an injection of £10 million (US$16 million) in its first formal round and is planning to move its gold nanoparticle delivery technology into phase II trials. The company will conduct two phase IIa trials for lead product, Midaform, in which insulin linked to gold nanoparticles is delivered via a patch inside the cheek. One trial will assess the patch in type 1 diabetes, the second in type 2. (See BioWorld Today, Nov. 6, 2013.)
