BB&T Contributing Editor
CHICAGO – The clinical diagnostics industry has become increasingly diversified in recent years as testing has moved from a laboratory-centered focus to encompass bedside testing in the hospital, physician’s office labs, home and self-testing, as well as tests performed in a wide range of specialized healthcare facilities such as ambulatory surgery centers, community clinics, dialysis facilities, pharmacies and nursing facilities. As an indicator of the breadth of sites in which diagnostic testing is now performed, the Centers for Medicare & Medicaid Services (CMS; Baltimore), which regulates all human diagnostic laboratory testing in the U.S., lists 25 different types of testing facilities in its categorization of the approximately 189,000 laboratory entities operating in this country, a statistic serving to demonstrate the widening decentralization of clinical diagnostics, as well as the pervasive role of diagnostic testing in the healthcare system.
Accompanying the decentralization trend, the complexity of diagnostic testing has also increased, particularly with the advent of genomics-based tests that measure arrays of molecular markers to produce diagnostic or prognostic information. The increasing complexity of diagnostic tests, coupled with the need to make test data available throughout the wide range of sites in which healthcare is delivered, is also driving demands for advanced information technology (IT) in clinical diagnostics.
The expanding role of IT in diagnostics is part of a broader trend in the healthcare system in which the use of information technology is driving a transformation in the delivery of healthcare. The role of IT in diagnostics, as well as in the overall healthcare system, was a major focus of discussions at the annual meeting of the American Association for Clinical Chemistry (AACC; Washington), held here in late July.
Point-of-care (POC) testing and its expanding importance in patient management was another topic highlighted at the conference. In particular, new developments in POC cardiac marker testing are having an important impact on patient management, and creating a significant growth opportunity in the market.
IT – reducing errors
As discussed by Harvey Fineberg, MD, PhD, of the Institute of Medicine (Washington), during the opening plenary session of the AACC conference, there is a significant opportunity for applying information technology to improve the quality of diagnostic testing, along with the quality of healthcare in general. Fineberg quoted data from the most recent study on laboratory errors, conducted in 1997, which showed an error rate of 0.045%, seemingly very low. However, that rate is about 100-fold higher than the six sigma defect rate targeted by a number of other industries and, based on the number of lab tests performed annually in the U.S. of seven to 10 billion quoted by Fineberg, translates into millions of lab errors per year.
Reduction of the error rate to a six sigma level would drop the number of lab errors to only thousands per year, resulting in a significant quality improvement. Achievement of six sigma error levels, however, will require implementation of new technologies, particularly IT-based technologies, as well as changes in incentives in the health care system. Fineberg noted that, at present, hospitals are paid more if they make a mistake, instead of having a financial incentive to reduce errors.
Pay-for-performance is thought to be one new initiative in the healthcare system that could help to improve the existing incentive structure. Fineberg said it is even more important, however, to adopt a comprehensive systems approach in diagnostics, since 85% of quality improvement is driven by changes in the systems employed to manage a process. Evolving to improved systems for diagnostics capable of reaching six sigma error rates implies the use of advanced IT, particularly in areas such as test ordering, result reporting, and result validation during the testing process.
Former Speaker of the House Newt Gingrich, who addressed the AACC audience in a special session on healthcare policy, also predicts an expanding role for information technology in healthcare, including diagnostics. Projecting that four to seven times as much scientific knowledge will be accumulated within the next 25 years as in the past 25 — including knowledge pertaining to health care — Gingrich said electronic health records and associated information systems for healthcare management will be essential to allow medical applications of that knowledge to be implemented effectively. A more interactive healthcare system is expected to develop that will be characterized by rapid evolution of best practices, including patient-directed care, and that will depend on a broad network of medical information that can be readily accessed by providers and patients.
Home testing, CPOE and EMRs
Gingrich predicted a shift toward prevention, early detection and patient self-management of disease, and this shift has created programs to eliminate cancer deaths through early detection, and others to improve the management of diabetes. In support of the benefits of greater decentralization of testing, Gingrich proposed that home testing be the norm in every case in which repetitive testing is performed, including wireless reporting of test results to allow patients to avoid the need for resource-consuming office visits. Computerized order entry, both for drugs and diagnostic tests, is another advance that should be more widely implemented, both to reduce medical errors as well as to improve efficiency.
Advanced informatics systems have so far had a limited impact in healthcare, due in part to a number of barriers to adoption. According to Richard Jones of the University of Leeds (UK) — discussisng the latest technological solutions for safe test ordering at the bedside at an AACC symposium on improving the clinical utilization of laboratory data — only a small percentage of hospitals have implemented computerized physician order entry (CPOE). A 2002 survey of U.S. hospitals found that only 10% had complete CPOE systems in place, and only a small percentage of lab tests are actually ordered via CPOE.
Companies developing CPOE systems and new informatics tools for clinical diagnostics include Cerner (Kansas City, Missouri), Anglia Healthcare Systems (Norfolk, UK), and iSOFT (Manchester, UK), which markets the LORENZO application software for healthcare information management.
One of the key barriers to widespread implementation of healthcare informatics is cost: A 200-bed hospital must spend at least $500,000 to install a system and about $175,000 per year to operate it. For a 1,000-bed hospital, installation cost is $1.5 million, and a similar cost is incurred annually for operation. Some hospitals that have installed CPOE systems have experienced a loss in productivity due to increased time spent on ordering.
While the LeapFrog Group (Washington), a consortium that is a leading advocate of the use of informatics to improve healthcare, estimates that CPOE has resulted in $2 billion in savings in the U.S. healthcare system; others, however, have estimated a cost of $6 billion and — taking a contrary position on the benefits — an increase in medical errors due to the difficulties of implementation. Clearly, computerization of healthcare is a long-term investment, with five to six years typically required to show a net positive return.
Jones said the transition to electronic health records (EHRs) and widespread use of informatics in healthcare will be patient-driven, and not the result of top-down national programs that attempt to force adoption of a certain model. He also predicts consolidation among the companies in the healthcare informatics market as the adoption of EHRs proceeds, citing the recent acquisition of Bayer Diagnostics (Tarrytown, New York) and Diagnostic Products Corporation (Los Angeles) by Siemens Medical Solutions (Erlangen, Germany) as an indicator of that trend in the diagnostics market.
Nevertheless, there are already examples of successful companies in the healthcare informatics market that have begun to integrate laboratory information into the electronic patient record.
Antek HealthWare (Reisterstown, Maryland), in partnership with Medical Communication Systems (Woburn, Massachusetts), has developed a laboratory information system called LabDAQ that interfaces with the MCS mMD.net EHR to provide a comprehensive medical information system focused on the physician’s office and small hospital environment. LabDAQ provides an electronic interface between both the EHR and laboratory analyzers, and can manage both in-house testing as well as tests sent out to reference labs. Antek leads the U.S. in installations with over 1,800, based on data from the most recent survey by the College of American Pathologists (Northbrook, Illinois). The system can serve sites ranging from small physician’s office labs up to a 250-bed hospital.
Antek has been particularly successful recently in the small hospital segment, achieving a 40% increase in sales to the hospital market in the last year. The MCS mMD.net EHR, which has more than 2,000 end users, is designed for ambulatory care sites such as physician offices where most medical care is delivered, and provides electronic prescriptions and test ordering, disease management and practice management functions, as well as portals for patients, providers and payers.
Another example of advances in informatics technology in diagnostics is the STELLARA information system developed by bioMerieux (Marcy l’Etoile, France). STELLARA is designed to integrate infectious disease testing in the central laboratory with the hospital pharmacy to reduce medication errors and reduce treatment time. It provides clinical decision support functions for the management of infectious disease, and also provides laboratory data management for coagulation testing and other lab disciplines. Along with bioMerieux’s latest systems for infectious disease testing, a package is now available for the hospital that can provide microbial identification and susceptibility test information within the same day.
The company has also partnered with HealthBlocks (Duncanville, Texas) to introduce the ENQ laboratory interface management system to hospital labs, which serves as a translator between laboratory systems to provide improved connectivity. The new informatics products are representative of bioMerieux’s strategy to transform itself from a supplier of lab equipment to an information company, reflecting an emerging trend in the diagnostics industry.
Expansion in point-of-care testing
The introduction of new information technologies, such as wireless information systems that allow capture of test data from an effectively unlimited range of sites, is enabling an expansion of diagnostic testing at the point of care, extending beyond the physician’s office to include sites such as patients’ homes as well as essentially any location within the hospital.
Craig Lehmann, PhD, of the State University of New York at Stony Brook, described a program using telehealth technology from Viterion TeleHealthcare (Tarrytown, New York) to monitor diagnostic test results and vital signs data from patients’ homes, with a focus on management of chronic diseases such as congestive heart failure and diabetes after discharge from the hospital. Demographic trends are resulting in rapid expansion of the elderly population in the U.S. and most other developed countries, creating a growing demand for healthcare services for the aged, which is delivered most cost-effectively in the home setting.
Lehmann presented data showing that the cost of care for those over the age of 85 is about three-fold higher than for the younger segments of the population and that the aged account for over 40% of all hospitalizations in the U.S. The high cost of institutional care is leading to a growing emphasis on home healthcare for the elderly, including those with serious chronic diseases. However, there is also a growing shortage of home healthcare nurses, with 500,000 unfilled job openings for nursing aides predicted by 2025.
One possible solution to both the labor shortage and the growing cost burden of home care is telehealth technology, which can allow a single nurse to monitor over 100 patients daily versus perhaps eight to 10 using in-person visits, at a cost of about $105 per month if the full range of POC monitoring devices is used. The cost for monitoring of glucose levels to manage diabetes with telehealth was quoted by Lehmann as 70 cents per day ($21 per month). The combination of information technology with POC testing to allow dissemination of care for chronic disease is expected to play a major role in care of the elderly, according to Lehmann.
Other companies involved in the home telehealth market include Philips Medical (Best, the Netherlands); Honeywell (Morris Township, New Jersey), via its HomMed unit; Alere Medical (Reno, Nevada); and Health Hero Network (Redwood City, California).
POC testing is also playing an expanding role in the hospital setting. As shown in Table 1, the market for POC testing products used to perform testing at the hospital bedside totaled almost $870 million in the U.S. in 2005, and growth is expected to significantly outpace growth of the overall clinical diagnostics market over the next five years, with sales exceeding $1.7 billion by 2011.
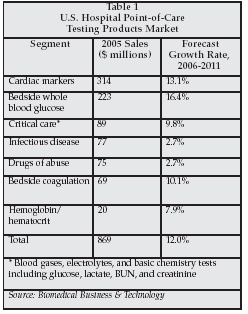
Major segments of the hospital POC testing market include critical care testing products (to perform blood gas/electrolyte and certain chemistry tests such as creatinine and BUN), coagulation testing products, cardiac marker tests, bedside whole blood glucose meters and test strips, rapid infectious disease tests, rapid drugs of abuse tests, and bedside hemoglobin and hematocrit testing products. High-growth segments of the U.S. hospital POC testing market include whole blood glucose and cardiac marker testing products, which are both projected to outpace growth in the overall hospital POC testing market.
Needed: tighter control
One factor driving growth in bedside whole blood glucose testing is the growing adoption of tight glycemic control (TGC) protocols aimed at reducing mortality and morbidity in critically ill patients, including both diabetic and non-diabetic patients. Recent clinical studies have shown an exponential decline in mortality in hospitalized cardiovascular patients with reduction in the average level of blood glucose. Three TGC protocols are in widespread use, including the Portland protocol, the Yale protocol, and the Leuven protocol, which employ intensive bedside glucose testing (often at hourly intervals) combined with insulin infusion to maintain glucose levels in a narrow range. A typical range is 85-110 mg/dL, as used in the Yale protocol, although individual hospitals may adopt wider or narrower ranges depending on their available bedside testing resources and their confidence in their ability to avoid hypoglycemic events.
The level of adoption of TGC protocols has grown rapidly in hospitals in the U.S. over the past three years. As shown in Table 2, data from the LifeScan (Milpitas, California) unit of Johnson & Johnson (New Brunswick, New Jersey), presented at the AACC conference by Glenn Johnson, VP of LifeScan’s Advanced Care Group, demonstrates that the number of U.S. hospitals that use LifeScan glucose testing systems and have adopted TGC has increased more than four-fold within the past two years. LifeScan estimates that 78% of the total LifeScan hospital base in the U.S. has now adopted TGC. A similar level of adoption is believed to exist in Western Europe, although the company does not have data for that region.

Adoption of TGC in other parts of the world is minimal, according to LifeScan, but there is growing interest in countries such as China. Patients in the intensive care unit, and particularly those with cardiovascular conditions, are typically the first group targeted for TGC protocols. However, of the 320 LifeScan hospitals that have adopted TGC, 17 now employ it hospital-wide, i.e., for all patients, whereas in a similar survey conducted by LifeScan in 2005, only one hospital employed TGC for all patients. TGC is increasingly becoming recognized as a standard of care for cardiovascular disease patients in the ICU. In fact, a number of suppliers of hospital blood glucose testing products, as well as some hospital users presenting at the AACC conference, indicated that groups such as the Joint Commission on Accreditation of Healthcare Organizations (JCAHO, Oakbrook Terrace, Illinois) may be adding TGC to the list of requirements that hospitals must meet in order to maintain accreditation.
TGC protocols also have economic benefits for the hospital, as discussed by Daniel Hilleman of the Creighton University School of Medicine (Omaha, Nebraska) at the AACC press conference. Recent released data from a 1,600-patient “before-and-after” study conducted at Stamford Hospital (Stamford, Connecticut) showed decreases in patient days in the ICU; ventilator days; total laboratory, pharmacy, and radiology costs; and post-ICU hospital length of stay, resulting in a net annualized decrease in costs of $1,580 per patient. Hilleman quoted other studies that have shown even greater cost savings. The cost to implement TGC is only about $87 per patient, according to Hilleman, allowing almost all of the cost savings of TGC to flow to the bottom line.
Logistical challenges
However, implementation of TGC can present logistical challenges, since nurses are required to test and download data for multiple patients 11 or 12 times a day. The use of paper-based reporting methods creates a high workload for the nursing staff and also leads to numerous errors in recording of data. As a result, the development of IT-based reporting systems, preferably wireless systems that can be used to provide data directly at the bedside, is a high priority.
LifeScan, in response to growing needs for improved POC data management in hospitals implementing TGC, introduced two new products at the AACC exhibition designed to improve transfer of blood glucose data between the point of care and the central laboratory. OneTouch DataLink Wireless provides wireless communication between LifeScan’s OneTouch Flexx meters and DataLink Workstations.
The second product, OneTouch DataLink Web, is used together with DataLink Wireless to provide connectivity from the bedside to the central laboratory. A key component of DataLink Wireless is a battery-operated wireless device server developed for LifeScan by Lantronix (Irvine, California), a leading supplier of networking technology including the WiBox wireless device server. The DataLink Wireless server transmits glucose test data from the Flexx meter and then powers down to conserve battery life, a key feature since the 802.11 industry standard data interface has high power requirements.
LifeScan also introduced a TGC program that in combination with DataLink Wireless and DataLink Web forms. LifeScan’s Integrated Intelligence, an integrated solution for tight glycemic control management. The TGC Manager program, which is now in use at Maine Medical Center (Portland, Maine), tracks nursing compliance, patient blood glucose levels in relation to targets, the number of patients and tests, and the time to reach the target range.
A number of additional POC testing products were introduced at the AACC conference, as shown in Table 3, addressing a wide range of applications from hospital critical care to POC nucleic acid tests for infectious disease.

Abbott Diagnostics (Abbott Park, Illinois) already a leading supplier in both the hospital POC critical care testing market with the i-STAT product line and the hospital bedside glucose testing market with the Precision PCx, introduced the Afinion chemistry analyzer targeting the physician’s office lab segment. The Afinion is manufactured by Axis-Shield plc (Dundee, Scotland) and will be distributed in the U.S. through Physicians Sales and Service/World Medical (Jacksonville, Florida). The initial application, a rapid hemoglobin A1c test, will target diabetes management, competing with the well-established DCA 2000 analyzer from Bayer Diagnostics/Siemens Medical.
Subsequent tests will also address diabetes management with an albumin-creatinine ratio assay to detect kidney failure, a common complication of diabetes; and hemostasis management, with a prothrombin time/INR test. Abbott plans to obtain CLIA-waived classification for all Afinion tests, thus addressing the more than 115,000 labs in the U.S. that only perform waived testing. Abbott also announced FDA clearance of a BNP test for the i-STAT analyzer, extending the menu to compete with the Triage POC cardiac marker system from Biosite Diagnostics (San Diego), although the i-STAT test is not yet CLIA-waived.
Another player in the POC physician’s office testing market, Abaxis (Union City, California), previewed the Piccolo Xpress analyzer, a new version of the tabletop Piccolo chemistry analyzer. Sales of the Piccolo instruments and reagent cartridges totaled $10.9 million for the fiscal year ended March 31, 2006, up 35% vs. FY2005. A typical physician’s office lab generates about $50,000 in on-going revenue for Abaxis annually, at 60% gross profit. The Xpress will address the growing demand for advanced information management technology in POC testing by providing increased data storage and a bi-directional interface. The Piccolo’s primary applications, accounting for 86% of the product’s sales in the POL segment, are lipid testing and comprehensive metabolic panels.
EpoCal (Ottawa, Ontario), a development-stage company run by the founder of i-STAT, exhibited a new POC blood gas/electrolyte system that will compete with Abbott’s i-STAT and a number of other POC critical care testing systems. The company’s EPOC analyzer employs smart card sensor technology, with all reagents and sensors contained in the card, thus avoiding the need for a complex and expensive analyzer. EpoCal also plans to add high-sensitivity immunoassays based on chemiluminescence technology to the EPOC menu. The company will launch its product first in the U.S., and recently filed for FDA clearance. A key differentiating feature of the system is room temperature storage of test cards for up to six months, greatly simplifying use of the product in POC settings.
Optical cartridge tech
HemoCue previewed a new WBC (white blood cell) test at the AACC conference that will be designed for CLIA-waived testing in primary care settings. The test is based on HemoCue’s proven optical cartridge technology, but employs a miniaturized camera and a staining reagent to count white blood cells from a capillary or venous whole blood sample. The product is expected to address the need for improved management of infections in the primary care setting, and particularly the over-prescribing of antibiotics, by allowing physicians to determine if the source of the infection is viral or bacterial during the patient visit.
Initial studies have indicated that the HemoCue WBC test shows good correlation with blood cell counters, which are accurate but often not available in primary care sites. HemoCue expects to file for FDA clearance for the system in the spring of 2007.
Another trend in the POC testing market, exemplified by the new HemoCue product, is the increasing sophistication of tests available for use at the point of care. The field has moved well beyond its initial applications in glucose and qualitative immunoassays for infectious disease to include high-performance immunoassays such as Troponin I and BNP, and most recently molecular testing. Cepheid (Sunnyvale, California) just introduced a Group B Streptococcus assay for its GeneXpert System, the Xpert GBS, that is designed for use by non-specialized personnel, requiring only four steps.
While the GeneXpert is not a portable analyzer for bedside use, its compact size and simplicity make it a potential choice for testing in POC sites such as clinics and other decentralized locations.
Furthermore, next-generation molecular diagnostic systems now under development may allow extension of molecular testing into an even wider range of sites. HandyLab (Ann Arbor, Michigan), an early-stage company founded in 2000 that is focusing on molecular diagnostics, exhibited a prototype analyzer designed for POC use, which employs microfluidics technology and will allow results to be generated in under one hour in essentially any setting.
The company is targeting market launch sometime in 2007. The initial application will be a Group B Strep test, followed by tests for Chlamydia trachomatis and Neisseria gonorrhea. HandyLab is just beginning clinical trials with the Group B Strep test. The system employs solid-state optoelectronics, an injection-molded microfluidic cartridge, fluorescence labeling, and real-time pcr amplification. In addition to a single-test version, the company also plans to develop systems allowing multiple (24 or more) tests to be performed at once.
